The Earth Invented Nuclear Reactors Before We Did
8:22 minutes
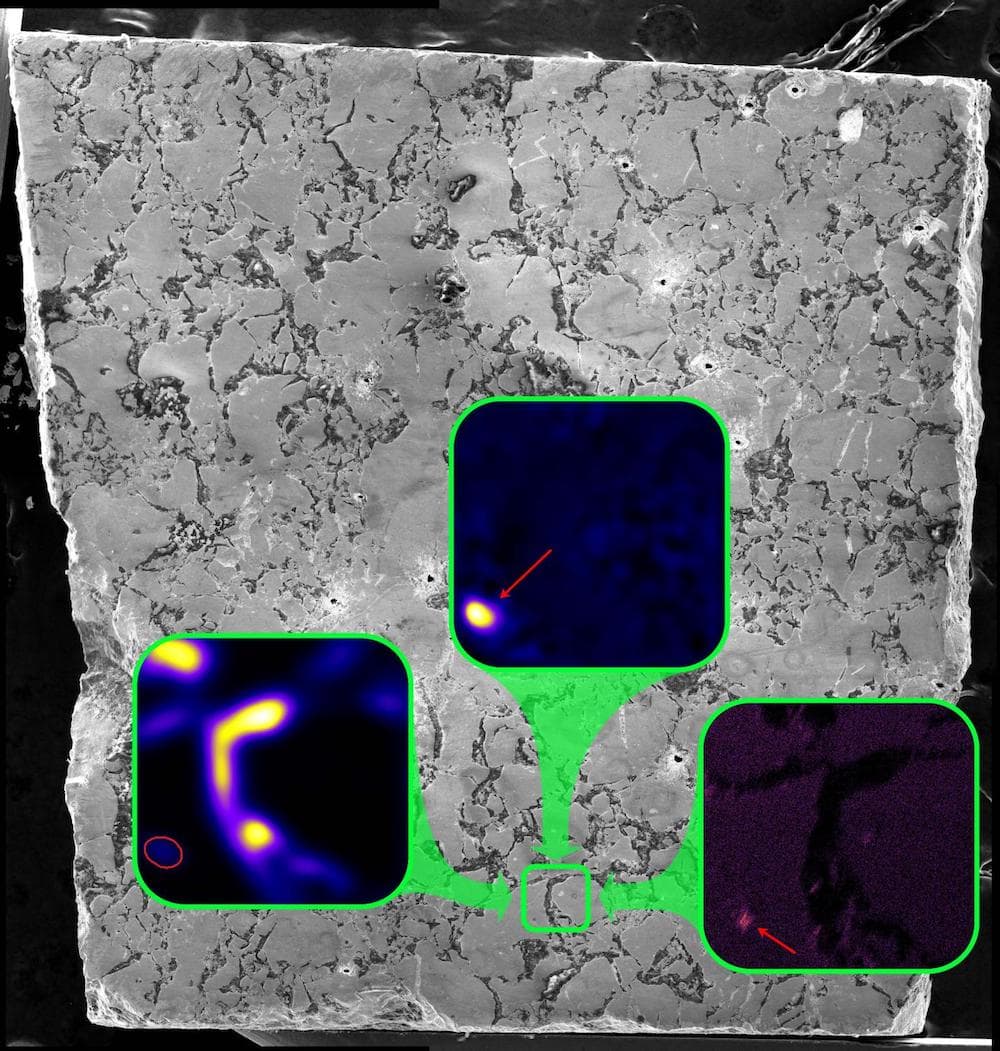
Long before humans enriched uranium to create nuclear fission, the Earth was doing it on its own. Two billion years ago, some natural deposits of uranium contained enough Uranium-235 to undergo spontaneous fission reactions. Those reactions cycled on and off for hundreds of thousands of years.
Those deposits are no longer undergoing fission. But, new research of the Oklo natural nuclear reactor in Gabon has found something curious. Not all the cesium (a toxic waste product of fission reactions both natural and man-made) was released into the environment. Rather, some remained bound in the reactor, with the help of other molecules.
Evan Groopman, a research physicist at the U.S. Naval Research Laboratory and author of the new research, explains why this finding could help lead to safer nuclear waste storage.
Evan Groopman is a research physicist at the U.S. Naval Research Laboratory in Washington, D.C..
IRA FLATOW: This is Science Friday. I’m Ira Flatow. You know, once we figured out how to do it, generating energy with nuclear fission is actually something simple to do. You just chuck some neutrons with enough energy at the right kind of uranium, or even plutonium, and you’ve created a chain reaction that can power a city or a bomb– sounds easy now, took a lot of time for people to figure that out. But you know who figured it out a long time ago? Mother Earth did.
There are deposits of uranium in the Earth’s crust where nuclear fission has happened naturally billions of years ago, when uranium deposits had more uranium-235 than they do now. These natural reactors even cycled on and off over hundreds of thousands of years. And they might be able to teach us something about safely storing waste for millennia to come. My next guest is the author of a new research published in the proceedings of the National Academy of Sciences looking at waste, at that waste. Evan Groopman is a research physicist at the US Naval Research Laboratory in Washington. Welcome to Science Friday.
EVAN GROOPMAN: Thank you, Ira, glad to be here.
IRA FLATOW: All right, I’m going to have to start off with the obvious question, how the heck did the Earth do this?
EVAN GROOPMAN: Right, I mean, you had an incredible confluence of conditions that happened in Gabon, Central Africa, two billion years ago. And so what you had was these natural nuclear reactors they were able to cycle on and off, as you mentioned, underground. And this happened for tens of thousands and hundreds of thousands of years.
So like you mentioned, reactors today need an isotope of uranium, uranium-235, to be enriched over its natural abundance. So its natural abundance right now is about 0.72%. And most of uranium, uranium-238, so 99% of it, can’t sustain these nuclear reactions. And so it turns out that two billion years ago, because uranium-235 decays six times faster than uranium-238, you actually had more uranium-235 two billion years ago. It was about 3%, or a little more. And this is about the same amount that you have in low-enriched uranium reactors today and allowed these reactions to occur.
IRA FLATOW: So why didn’t one go critical and explode, turn into [INAUDIBLE]?
EVAN GROOPMAN: Right, so there’s a couple of other conditions that you need. First, you need enough of this uranium packed together. And then, you also– generally, you need a neutron moderator. And so ground water around these reactors actually moderated the neutron. So uranium-235 likes to fission when it has slow or low-kinetic-energy neutrons. Normally, during a fission event, you split an atom into two roughly equal mass halves, and you also have some neutrons. These neutrons are very energetic.
And so collisions with the water that was coursing through this reactor actually allowed– it slowed down the neutrons enough to split the uranium and keep this fission process going. And so this water also ended up moderating the reactors and prevented a runaway, because when the reactors are running, they heat it up a bit, and they turned the water into steam. And this prevented the reaction from continuing. And so after about 30 minutes, the reactor turned off and stopped this nuclear chain reaction until it cooled down enough for the water to seep back in and the reaction started up again. So it had this self-control mechanism that allowed it to cycle.
IRA FLATOW: And so how do you find– what is left behind so you know that something like that has happened? How do you search for it?
EVAN GROOPMAN: So these things were discovered by a French worker who, in the 1970s, was looking at these uranium ore deposits that were being mined. And it’s really a triumph of mass spectrometry that they were looking at the ratio of the two isotopes of uranium, 235 to 238, and he noticed that this ratio was slightly lower than it should be. So he measured a ratio of 0.717, and it should have been 0.72, or thereabouts. And it sounds like a very small amount, but it turns out that this is actually a large variation that you see across the entire Earth and the whole solar system.
So they knew that something was up, and they went looking for the specific places where this uranium-235 was even more depleted. And when they picked up this ore from these regions, they then were able to fingerprint that nuclear fission had occurred by looking at the fission products. So these are these two halves that split apart from a uranium atom. And you look at different elements of these, such as neodymium, and the distribution of the isotopes of these elements that formed from fission are very different than you find elsewhere on Earth. And so this provided the fingerprint so you knew, OK, it had to be nuclear fission.
IRA FLATOW: Interesting. We all know that the nuclear reactors produce waste. And that’s really a major problem we have now with them, is what to do with the waste. So what happened to the waste from these reactors, and can we learn anything from it?
EVAN GROOPMAN: Well, these reactors are very interesting because they’re really our only natural analog. And they’re the best analog we have for studying the retention of waste long term. And because there was water coursing through these reactors, and there’s about 17 reactor sites in this region– so you had a different level of processing and different types of processing that were going on to these reactors in their 2-billion year history since they stopped working. So there was groundwater. There was local volcanism that heated up rocks.
And so when you go in and look at the different elements and isotopes that are in these different regions, you can tell how much of each element was lost or retained at the reactors. And so that was some of the work that we did. We started looking around, and we found that cesium, which is one of the elements produced in these fission events– and there’s several different fissionogenic isotopes that are produced of cesium. It was actually retained in certain minerals within these reactors.
So these were ruthenium metal that formed inside the reactors. And it captured this cesium while it was still radioactive and then retained it for 2 billion years after the reactor stopped. And this is really remarkable, because cesium is a very mobile element. It’s volatile, which means when you heat it up, it moves around a lot. And it’s also incompatible with the uranium fuel crystal structure. So it’s lost very easily from the reactor. And so it was incredible to actually see that it had been captured in these new minerals.
IRA FLATOW: So it’s locked up, so to speak.
EVAN GROOPMAN: Right, exactly.
IRA FLATOW: Interesting. So you don’t think, because it’s now been 2 billion years and all this uranium has decayed, that there are any active natural reactors still around.
EVAN GROOPMAN: That’s correct. There aren’t. And it’s because the uranium-235 isn’t enriched enough anymore.
IRA FLATOW: Mhm. So where do you go from here? Well, let me just say, these were found in mines, right? People were in mines looking for– were they mining or looking for them?
EVAN GROOPMAN: So they were mining uranium. They knew that uranium was there. And some of these mines were on the surface. Open-pit mines and others are about 200 meters below the surface.
IRA FLATOW: Wow. So what’s next for you with this project?
EVAN GROOPMAN: So now that we know that ruthenium, for instance, and some of these minerals capture cesium and other fission products, one avenue of research is to figure out if we can create new materials that hold these fission products better. Or when you’re thinking of long-term storage, one thing that’s done today is you try to vitrify, or turn, uranium waste into glass, or you add it to glass so it’s retained. And now that we know that ruthenium actually captures the cesium, and ruthenium is present, it’s made in the nuclear fuel by these reactions, perhaps we can modify the chemistry a bit to force that cesium to be better retained in these long-term waste structures.
IRA FLATOW: Wow. That’s very, very interesting. Thank you for taking the time to talk with us today.
EVAN GROOPMAN: Thank you.
IRA FLATOW: Evan Groopman is a research physicist at the US Naval Research Laboratory in Washington.
Copyright © 2018 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Christie Taylor was a producer for Science Friday. Her days involved diligent research, too many phone calls for an introvert, and asking scientists if they have any audio of that narwhal heartbeat.