Stretching The Boundaries Of Cell Biology
17:08 minutes
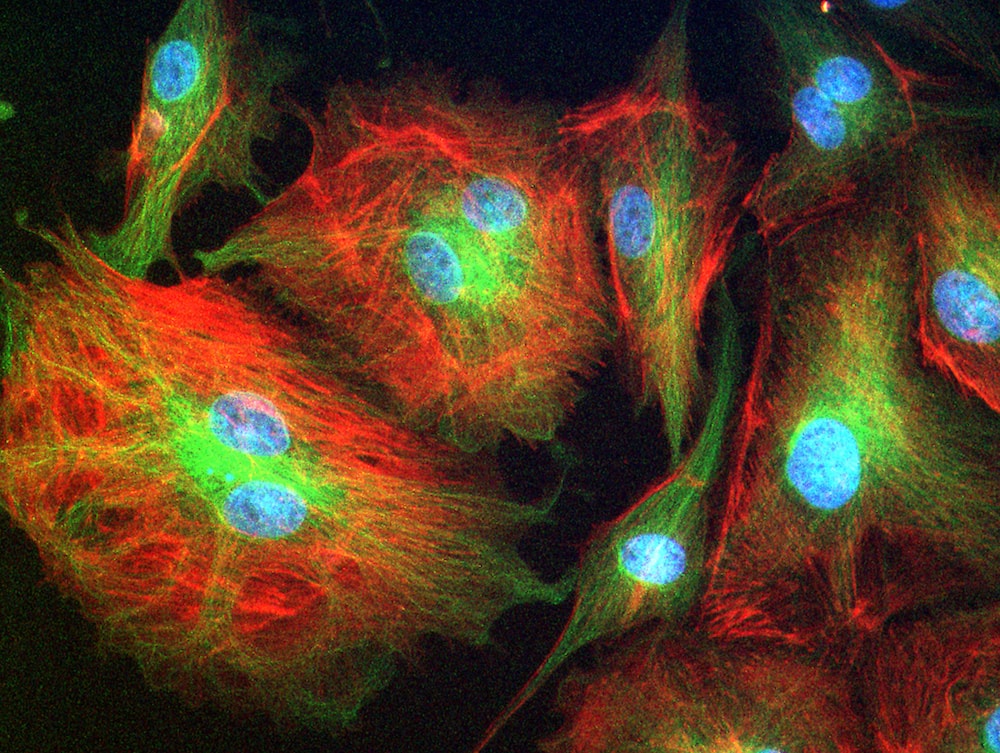
Throughout their lifetimes, the cells in our bodies have a number of decisions to make. Should they divide and multiply? If they’re stem cells, what kind of tissue or cell should they become? And, sooner or later, should they die? In order to make these decisions, cells rely on both genetics and chemical signals from other cells.
But what about physical signals? Pushes, pulls, and whether—or how hard—other cells are touching them? As it turns out, there’s a lot of mechanics involved in cell biology as well. This isn’t entirely new information. Thanks to century-old research, women at risk of osteoporosis are often advised to lift weights: the loading encourages their bones to deposit more cells and become more dense.
But as medical technology advances, and bioengineers find new ways to play with the forces cells encounter, researchers are learning more about how we can manipulate physics to repair tissues, steer stem cell developments, and even understand and treat cancer.
David Mooney, a professor of bioengineering at Harvard, explains some of the common mechanisms that we’re just beginning to understand, as well as ideas for therapeutic devices that are now coming out of mechanobiology.
And Dana Damian, a lecturer in bioengineering at the University of Sheffield in the United Kingdom, explains her robot that could someday help treat conditions in which tubular organs, such as the esophagus or bowel, are too short—just by gently stretching them.
Dana Damian is a lecturer in Automatic Control and Systems Engineering at the University of Sheffield in Sheffield, United Kingdom.
David Mooney is a professor of Bioengineering at the School of Engineering and Applied Sciences at Harvard University in Cambridge, Massachusetts.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. Now, what if I told you, you could make your cells grow, divide, expand, even die, by simply pushing or pulling on them. You’d say, hey, I thought that was the whole role of DNA, right– signaling when cells should do that? Now, I would say you’ve already seen it happening around you– skin growing, expanding to accommodate a pregnancy, right? More skin– bigger belly. Your doctor says, hey, if you want to avoid losing bone mass, osteoporosis– go lift some weights, encourage new bone growth. So scientists, physicians, have known about this mechanical stimulation for many decades, but only recently has the technology of smart robotics been brought into the picture.
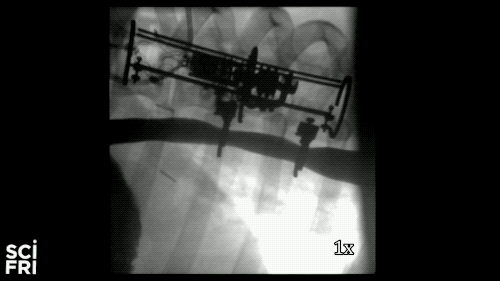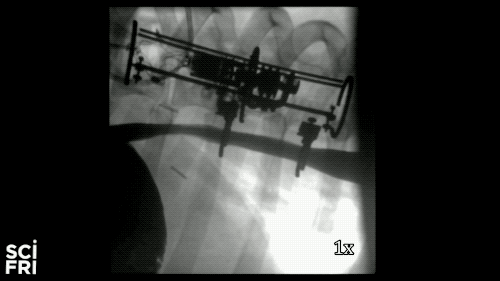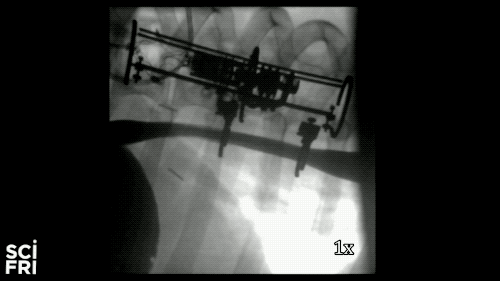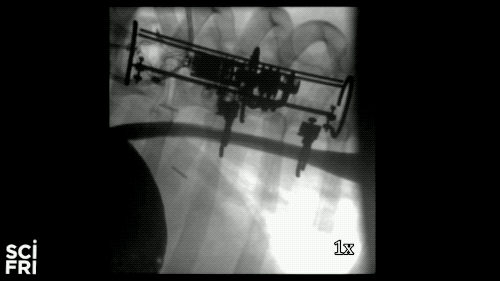
Here’s a case in point. A new robot described in the journal Science Robotics this week, sets out to harness the power of pulling. When fastened along a pig’s esophagus over a period of nine days, the robot gently stretched the tissue by more than 10 millimeters– that’s 10 millimeters in nine days– much of that from cell division, alone. So for babies born with rare birth defects called esophageal atresia, this could make a real difference in their lives. So why exactly did this work and what kinds of medical interventions could we perform with pushing and pulling or prodding robots? Well, that’s what we’re going to be talking about this hour. You want to join us– 844-724-8255. 844-SciTalk. You can also tweet us at @scifri.
Let me introduce my guest– Dana Damian, a lecturer, Director of the Biomedical Robotics Lab at Sheffield University in the UK, one of the creators of the esophagus tugging bot. She joins us by Skype. Welcome to Science Friday, Dr. Damian.
DR. DAMIAN: Hello. Hi, thank you. Thank you for this invitation.
IRA FLATOW: You’re welcome. And David Mooney, Professor of Bioengineering at Harvard in Cambridge, welcome, Dr. Mooney.
DR. MOONEY: Thank you. It’s my pleasure to be here.
IRA FLATOW: Dr. Damian, when I hear, “robot,” I think as something that walks around and maybe talks– that’s not quite what you’re describing, though. Describe it for us, please.
DR. DAMIAN: That’s true. Yes, as a matter of fact, in robotics we are very much used to see robots that manipulate, robots that walk, just as you said. So I think where we’ve been taking a little bit of a different approach, because, in the body, it’s not so much about manipulation– well, actually, it a bit of manipulation– it’s not so much about walking, especially at the scale of a centimeter. What the body really does is, or the action that usually take place in the body is a lot about pressure and fluids running through organs, through the body.
IRA FLATOW: Tell us about what you did in the esophagus– you put two rings in there, in the esophagus?
DR. DAMIAN: Yes, yes. Yes, so we have this robot that attaches to an esophagus using two rings. The robot is equipped with sensors– [? four ?] sensor, a position sensor– and so we’re able to measure how long we are displacing the tissue or how much force we apply to the tissue at any time during the treatment. And then, just as you said, we have we have a [? monitor ?] that can apply gentle forces on the tissue of desire values, intensities or signed [INAUDIBLE]. And so what we’ve done, we’ve applied about 2.5 millimeter tissue displacement per day over nine days, and we were demonstrating in vivo trials with swine animal that we elongate the tissue more than 77%.
IRA FLATOW: Why does this happen? Do we know?
DR. DAMIAN: Well, there’s a lot of things that we don’t know, for sure, and maybe David will have his better explanation than I do, but it does look like mechanical tension is a powerful driving force in the physiology of the tissue and the signal that cells receive through this mechanical stimulation can regulate the [? fate ?] of the cell.
IRA FLATOW: David, Dana has been telling us about this tissue– it’s not stretching it, it’s actually making new cells grow. Correct? Well,
DR. MOONEY: So I think the two things are related. So by applying a stretching, she’s then able to induce a growth of the tissue.
IRA FLATOW: Mm-mm. And what does that tell us about what we thought about how cells reproduce?
DR. MOONEY: Yeah, so I think it’s actually a really exciting observation and finding, and certainly has great potential, clinically, to help a variety of different types of patients, including these children that you had mentioned earlier. On the one hand, it extends what we already know. So as you mentioned in your intro, we’ve long appreciated that physical forces regulate a lot of biology. If you think about it, the cells in our bodies live in a very physical world. We walk around, gravity is always pulling on our tissues, there is blood flow through our heart and the vessels. So it makes sense that the cells would respond to these environmental signals and alter how they grow, how they die, how they specialize, and what kinds of functions that they might have. But what’s really new here, is now the extension of a lot of this basic knowledge into the ability to now apply defined mechanical signals and to try to drive regeneration and growth of new tissues.
IRA FLATOW: And so this could possibly have an effect on people once this is as, Dr. Damian was saying, this has been proven– we were showing it in pigs here, but now it might work in people? I mean we might be able to do that?
DR. MOONEY: Absolutely.
IRA FLATOW: Tell us how. Give me some examples.
DR. MOONEY: Yeah. Well, first of all, I’ll actually put this in little bit historical context. So we already do some of this already, even though maybe oftentimes people don’t think about it. Many of us have children that we’ve taken to an orthodontist and had some procedures done, in terms of the braces. And when you apply braces, you’re also applying a physical force on the tissue– in this case, the tooth structure– to get not just the teeth to move, but the bone underneath those teeth to actually remodel. In the area of wounds, we currently have some therapies where people who have significant wounds that aren’t healing well on their skin, we apply vacuum in the clinic and basically pull on those tissues to try to enhance healing.
So there certainly is precedent for this that’s out there. And so what I think the exciting thing here is the ability to now take this into the body and use these soft robotic systems that are very different than the hard robots that I think most of us think about from the movies. And now be able to use those in the body because they can apply very gentle, very reproducible forces on the cells and the tissues to induce this type of remodeling and regrowth and regeneration. And not only can we do it outside the body, but we potentially actually, inside the body, we can potentially have these devices outside to apply these types of loads, as well. So you know, we have soft materials, and we can place these in the body surgically. We can wrap them around tissues in the body, and then program them to deliver the kind of forces that we’ve learned are important and can drive regeneration.
IRA FLATOW: Dr. Damian, how hard is it? We hear Dr. Mooney talk about putting a robot in the body– how hard is it to design a robot that’s supposed to function inside the human body?
DR. DAMIAN: It is quite challenging and it truly depends on the medical– on the clinical condition that we are targeting to resolve. We’ve been going through a lot of trials and uncertainties in designing this robotic infant and tell you the truth, when I first saw it actually working, I felt like I’m controlling a rover on Mars just because it is in an inaccessible place. And so one once we place it there inside, we need to make sure that it is going to work 100% of the time. And I think this is a huge– this is a huge challenge. We need to go through many stages of design. So how can we design such a robot that takes into account, not only the targeted tissue, but also the environment around? So we have soft tissue, like the esophagus, like the lungs– but we also have hard tissue– speaking about the robotic infant that we’ve developed– such as ribs. And so we want to have a robot that is both soft, but is also durable.
IRA FLATOW: Right.
DR. MOONEY: And well, it took us quite a while to understand all these uncertainties in order to embed the requirement in this design. So we’ve come up with an encapsulation that is soft, it’s wrinkled– in order to take into account that gentle interaction with the soft tissue– but we also had to embed polyester mesh inside it just in order to take into account the stress from the ribs.
IRA FLATOW: Right.
DR. DAMIAN: –which could [? tear ?] the encapsulation at any time, and that would be so devastating for both the human or the animal, as well as for the robot, because we have a lot of electronics inside which can oxidize or which can short, just because there’s going to be some fluid running into the [? unit. ?]
IRA FLATOW: Dr. Mooney, people are going to listen–
[INTERPOSING VOICES]
IRA FLATOW: I’m sorry, people are going to listen to this and say, hey, I have a child who’s suffering from this or that or I have a friend– this is not ready for humans yet, is it?
DR. MOONEY: Not the type of intervention we’re talking about today, but as in many types of advances in medicine, there will be a stepwise transition. So to put these devices in the body, as we just heard, has a lot of challenges, and there will have to be a lot of work done to make sure that can be done safely and reproducibly. But if, for example, we instead are applying these outside the body, it actually lowers the bar and the hurdles to do it substantially. And that’s something that you have the opportunity to move much more quickly, I think, towards human clinical trials. If you think about it, today, many people already get massage therapy, which is, in some ways, similar to what we’re talking about today– where an individual pushes and pulls on tissues to try to alter. Now in massage, we typically don’t know exactly what it is we’re manipulating, and it’s also difficult, if not impossible, to have a defined and repeatable force done over and over again, multiple days. But with a soft robotic that you place outside the body, you can accomplish that. So how I see this playing out is we’ll have devices outside the body, initially, that can induce regeneration of certain tissues– probably not the ones deep inside body organs– and then, as those get developed and go to the clinic, then we’ll be continuing to make advances on these internal soft robotic devices.
IRA FLATOW: How would this interact with stem cell research? What you’re saying, reproducing cells, is a lot of what STEM cell research is aimed at. Can this robotic idea– the pushing and pulling– do away with some of the need to use stem cells in some certain cases?
DR. MOONEY: Yeah. So it’s a really provocative idea that instead of culturing outside the body and transplanting stem cells, which is how most of us think about regenerative medicine happening today, to instead directly target those cells in the body that already exist. And, for example, as you’re saying, apply a certain stress to induce them to proliferate and then have them specialize and become the tissue type of interest. There is proof of principle for that already. For example, in the area of skeletal muscle, it’s been demonstrated that one can induce regeneration of muscle, which is caused by a stem cell population simply by applying mechanical cues. And what we’re talking about with Damian’s work, at this point, it’s not completely clear if there is a stem cell contribution. But at the end of the day, there likely is at least some stem cells that are participating in the building of the bulk of the tissue where these blood vessels. So likely, stem cells are being targeted there. And we have found in the lab, many groups that stem cells are exquisitely sensitive to these types of physical cues.
IRA FLATOW: I’m Ira Flatow. This is Science Friday from PRI, Public Radio International, talking with Dr. Dana Damian and Dr. David Mooney talking about mechanically manipulating cells to get them to move. And what about in cancer cells? If we push and pull cancer cells the right way– might we disable them?
DR. MOONEY: That’s actually, again, a really striking concept. If you think about it, how we oftentimes detect cancer– if you think about breast cancer– when a woman does a self-exam, she’s actually looking for a region of the breast tissue that’s stiffer. So we intrinsically know that there’s a different mechanical environment for cancer, and people have appreciated this for a long period of time. So that naturally led to the question of whether cancer– whether this stiffness is a result of the cancer, or perhaps it actually causes the cancer? And over the last decade or so, there’s been a tremendous amount of work that’s shown that the mechanical environment of cancerous cells plays a really dramatic role in their further development of malignancy– their ability to move and migrate and colonize other parts of the body. And so that actually is an area where there’s a lot of research now to try to alter the mechanical environment of cancer cells to try to, in essence, either prevent them from being able to metastasize or perhaps even return them back to a normal state.
IRA FLATOW: Let me see if I can get–
DR. MOONEY: So that’s–
IRA FLATOW: That sounds–
DR. MOONEY: Oh, no, please, go ahead.
IRA FLATOW: Sounds exciting. I just want to see if I can get a call in from a listener before we have to go. Quinton in San Antonio. Hi, Quentin.
QUINTON: Hi. Appreciate it.
IRA FLATOW: Go ahead.
QUINTON: I’d like to ask– you said the experiment lasted for nine– eat and function normally or if it required IV fluids.
IRA FLATOW: Yeah, he sort of dropped out a little bit. So you’re asking was the pig able to eat and function normally with the robot on?
QUINTON: Yes.
IRA FLATOW: Dr. Damian?
QUINTON: Yes, ma’am. Yes, sir.
DR. DAMIAN: Yes, I heard that. Yes. The short answer is, yes. And the long answer, to explain it to you better– we’ve mounted that robot on a healthy esophagus, so [? non-interrupted ?] esophagus. And so we would have a normal tube, basically, and while the animal can keep on moving and eating and drinking water, we’re doing– or the robot is doing– the job that it’s supposed to do.
IRA FLATOW: And can you give us other kinds of illnesses that you might, or other different kinds of tissue you might aim, Dr. Damian?
DR. MOONEY: Sure, sure. So we have targeted, currently, the esophagus because it’s a rather simple soft tissue. It has mostly wall and the transportation of the food from the mouth to the stomach. And it has some muscular layers there in order to transport the food. But we’re also looking now at the short bowel. There is this devastating condition called, “short bowel syndrome,” where children are born with a shorter bowel. So that means they have an impaired digestion. And so we have– from our preliminary trials, it looks like we can also elongate this tissue.
IRA FLATOW: And for adults who have bowel shortening surgery? Might this?
DR. DAMIAN: Yes, it looks like this could also apply, yet this is something that we still have to demonstrate.
IRA FLATOW: Wow. This is quite interesting. You know, as I said before, this is not a new finding. We know– we’ve known for decades about this happening, yet it’s now coming to the fore right now as new research is published about how successful you researchers have been. I want to thank both of you– Dana Damian, Lecturer, Director of the Biomedical Robotics Lab, Sheffield University in the UK and one of the creators of the esophagus tugging bot, and Dr. David Mooney Professor of Bioengineering at Harvard in Cambridge. Thank you both for taking time to be with us today.
Copyright © 2018 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Christie Taylor was a producer for Science Friday. Her days involved diligent research, too many phone calls for an introvert, and asking scientists if they have any audio of that narwhal heartbeat.