A Nobel Prize For Chemistry Work ‘Totally Separate From Biology’
23:55 minutes
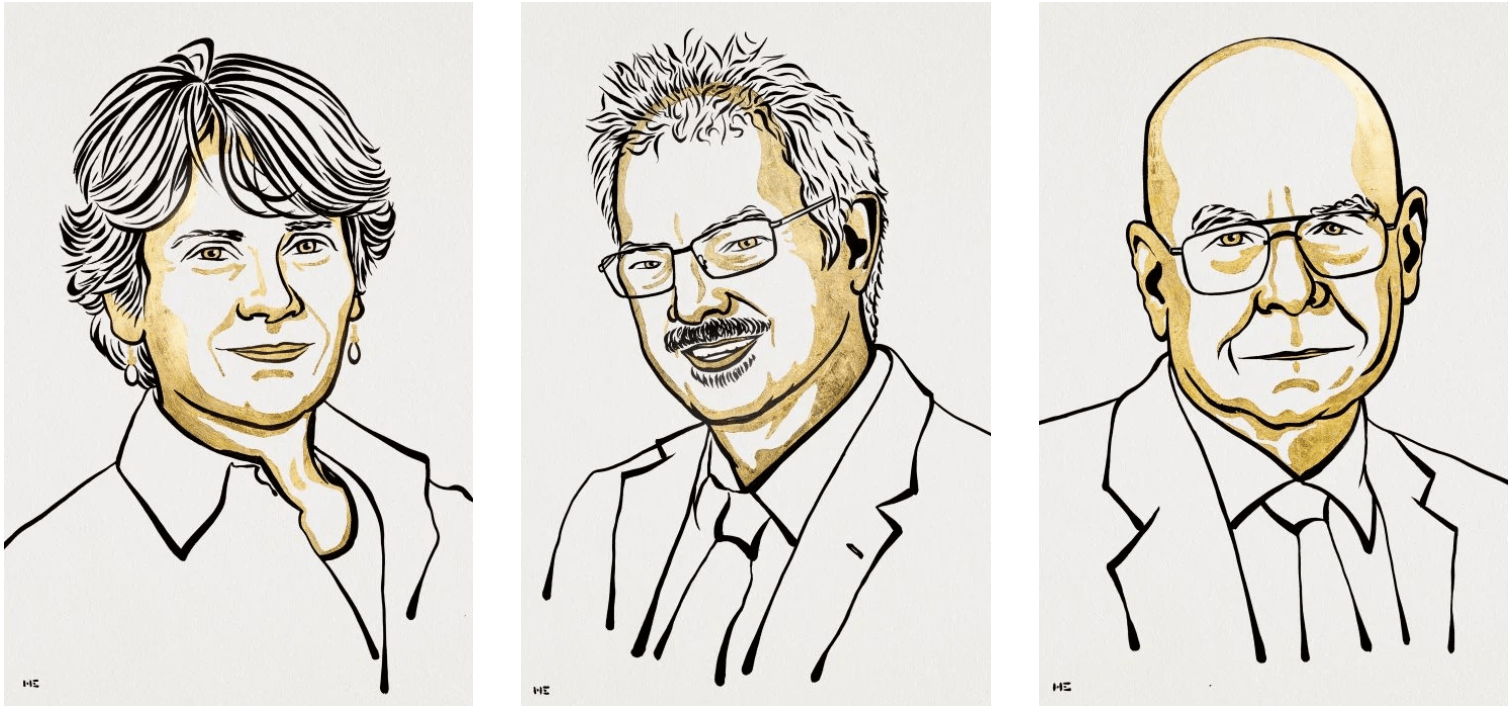
 This year, the Nobel Prize in Chemistry went to Carolyn Bertozzi of Stanford University, Morten Meldal of the University of Copenhagen, and K. Barry Sharpless of the Scripps Research Institute “for the development of click chemistry and bioorthogonal chemistry.” In “click chemistry,” molecular building blocks snap together quickly and efficiently to let chemists build more complicated molecules. But bioorthogonal chemistry takes that work one step farther, allowing the technique to be used within living organisms without damaging cells.
This year, the Nobel Prize in Chemistry went to Carolyn Bertozzi of Stanford University, Morten Meldal of the University of Copenhagen, and K. Barry Sharpless of the Scripps Research Institute “for the development of click chemistry and bioorthogonal chemistry.” In “click chemistry,” molecular building blocks snap together quickly and efficiently to let chemists build more complicated molecules. But bioorthogonal chemistry takes that work one step farther, allowing the technique to be used within living organisms without damaging cells.
“When someone is thinking outside the box, or in a very different way, we like to think of that as orthogonal thinking,” Dr. Bertozzi explained. “So biorthogonal means not interacting with biology. Totally separate from biology.” Her research began with an interest in developing ways to see specific sugar molecules on the surface of cells. But it has developed into an approach that can be used for advanced drug delivery in fields such as chemotherapy.
Bertozzi joins Ira Flatow for a wide-ranging conversation about her research, chemistry education, her early music career, and the importance of diversity in the field of chemistry.
Invest in quality science journalism by making a donation to Science Friday.
Dr. Carolyn Bertozzi is a 2022 Nobel Laureate in Chemistry and a Professor of Chemistry at Stanford University in Palo Alto, California.
IRA FLATOW: This is Science Friday. I’m Ira Flatow.
If you think about it, your body is a bit like a crowded party. Yeah, it’s packed with sugars and proteins, ions, nucleic acids, and a whole lot more, all mingling in the same place. And that poses a big challenge for biology researchers. Because if you create a drug whose molecules mingle with the wrong guests at the party, that could cause a harmful side effect.
To combat the issue, scientists have invented a whole new class of chemical reactions, called bioorthogonal chemistry. These reactions involve two chemicals that can bond with each other even in an environment as complicated as the human body. And they don’t interfere with the normal chemical reactions in the body. Today, bioorthogonal chemistry is a staple in biological research and a promising tool for medicine. It’s been so impactful this year, its pioneer won the Nobel Prize in Chemistry for her research.
Let me bring her on now. Dr. Carolyn Bertozzi, 2022 Nobel Prize Laureate and professor of chemistry at Stanford, in Palo Alto, California. Congratulations, and Welcome to Science Friday.
CAROLYN BERTOZZI: Thank you so much. It’s really wonderful to be here.
IRA FLATOW: Nice to have you. OK, let’s start with the basics. What does it mean for chemistry to be bioorthogonal? A pretty big word there.
CAROLYN BERTOZZI: Yes, it’s a bit of a mouthful. But you can break it down into its two parts. So the word orthogonal is one that we usually use when we’re trying to describe two things that don’t interact with each other, right? When someone is really thinking outside the box or in a very different way, we like to think of that as orthogonal thinking. So bioorthogonal means not interacting with biology. Totally separate from biology.
And we invented that concept because we had ideas for how such a chemistry could be useful in biology and medicine, as you so nicely put in your introduction.
IRA FLATOW: Well, I just gave an overview, but give me your idea of how useful it is.
CAROLYN BERTOZZI: Well, it started with a very specific application in mind. And what the back story is is that I had a longstanding interest in the biology of complex carbohydrates. And people might think of that term as having to do with food that you eat. But there’s a whole different world of biology of complex carbohydrates. Which is that they’re basically like a forest that decorates the entire surface of every cell in your body. And we wanted to be able to study those complex carbohydrates. And we wanted to image them. We wanted to be able to see them in microscopes or see them in a magnetic resonance imaging scan or a PET scan. And there was no way to see them.
So the very early genesis of bioorthogonal chemistry was as a tool to be able to study and to visualize those cell surface sugar molecules.
IRA FLATOW: And why do you want to know so much about how the sugar molecules are working on the cell surface?
CAROLYN BERTOZZI: Well, even back in the 1990s, which is when I started this whole area of research, people knew that the structures of cell surface sugars change during diseases. And the area in which this had been studied most extensively was in cancer. So people knew that if you looked at the surface of cancer cells, the sugars had changed compared to the normal cells around them. And any time there’s a change in molecules between healthy tissue and cancerous tissue, you might be able to exploit that as a means to visualize those disease cells and to detect the disease.
But the problem was, at that time, the only way to study those sugars was on cells and tissues that you took out of the body and ground up into bits and pieces and destroyed. And there was really no way to look at the sugars on the cells when they were alive and in the body. And that’s what you need to do if you want to be able to detect these changes for cancer detection or cancer diagnosis.
IRA FLATOW: And so how does looking at sugars connect to the idea of this orthogonal model? How did you apply it?
CAROLYN BERTOZZI: Well, it’s funny. Because science usually has a back story of accidents and serendipity. And this story has an element of that. So way back when I was a postdoctoral fellow– and so this is before I was a professor; instead, I was a researcher in someone else’s lab at the time– and I was thinking about the sugars, and frustrated that there was no way to visualize them. And other molecules, like proteins and DNA and RNA, other stuff that you find in cells in animals and humans, there were actually some really nice technologies to look at those other molecules. But there was just a gap, there was nothing for the sugars.
And then I happened to go to a conference in Southampton, England, of all places, as a postdoctoral fellow, and I heard a lecture from a German biochemist, named Werner Reuter. And in that lecture, he talked about the fact that you could feed cells chemically altered versions of very simple sugars. And as long as the alterations were very subtle– not too dramatic– those altered sugars would get metabolized by the cells and they would be incorporated into the cell surface complex carbohydrates.
So you could actually make the cells turn into what they eat. You’ve heard the phrase, you are what you eat, right? And these cells actually were putting altered sugars on the surface just because they were eating the little simple precursors. And so that gave me an idea for how you could sneak a little bit of chemistry into cell surface sugars, and then use the chemistry to attach probe molecules for imaging.
And it was a very simple idea at the time. But there was a huge problem with the idea because you would need the simple chemistry to be what we later termed bioorthogonal. And there really wasn’t a chemistry that existed at that time that would allow you to do such a thing. So that was where the motivation came from. And if I hadn’t seen that lecture by Professor Reuter, I’m not sure I would have had this idea any time soon.
IRA FLATOW: Well, how do you guarantee that your bioorthogonal chemistry is only going to react with the one specific thing you want it to?
CAROLYN BERTOZZI: Well, that is the central challenge in the whole area, is to find these magical chemistries that, even though there’s thousands of other chemicals in your body, that somehow they’re willing to ignore all of that and yet still react with something else that’s bioorthogonal. And we spent many years engineering the chemical groups so that they would have exactly this sort of thread-the-needle capability. And you have to do a lot of experimentation.
So when we made new functional groups, which is what we call chemical groups, well, we would test them. We would test them in cells in a dish, first and foremost. And we would do a lot of experiments to see whether they were attaching themselves unwittingly to some of the biological molecules. And if they did, we threw them in the garbage can and went back and tried a different type of chemical.
And once we got them to be very clean in cells, then we would test them in animals. And enough of that has now transpired over the last 25 years that we now are very confident that we have a toolkit of bioorthogonal chemistries that are so safe that some of them are now being tested in human clinical trials. They’re inside the body of human patients.
IRA FLATOW: Well, that brings me to the meat of my next question, so to speak. Give me some ideas of how you might apply this chemistry.
CAROLYN BERTOZZI: Well, right now, the two leading applications have to do with drug delivery, especially for cancer. And we all know that the types of medicines people are traditionally treated with for cancer, which we call chemotherapies, they can be really toxic and hard to handle. And the way that those medicines work is they kill cancer cells, but they also kill some of your healthy cells at the same time. And that’s why people on chemo have these side effects, like losing their hair and being very nauseated, and sometimes having organ damage. And on a bad day, the drug might do more harm than good to a cancer patient.
So one of the challenges is to figure out, how do you send that toxic drug to the cancer cell specifically, like a guided missile, and keep it away from all the other cells in the body? And bioorthogonal chemistry has turned out to be useful for this. And so as a case in point, there is a biotech company here in the Bay Area outside San Francisco that I am an advisor for. And they have a protocol to treat patients with soft tissue sarcoma. That’s a particular type of cancer in bones.
And the way they do this is they inject into the tumor area a material. It’s a hydrogel polymer, and it’s actually quite similar to the same material that’s injected cosmetically for people who want filler. If you’ve ever heard of cosmetic fillers, there’s literally a polymer that gets injected into the face, where a person wants to fill a wrinkle, for example.
IRA FLATOW: Yeah. We’re not talking Botox here, right?
CAROLYN BERTOZZI: No, not Botox. This is a different substance. It’s harmless. It just kind of puffs up your skin.
So what this company has done is they have modified that same material with a bioorthogonal chemical. And they inject that material into the tumor. And it does nothing but fill up space. It’s harmless. That bioorthogonal chemical has no interaction with the human body. It’s just sitting there. But then, the next day, they inject the chemotherapy drug systemically. They put it, in the typical way, in an IV bag. So the patient sits there and they have an IV infusion of chemotherapy.
But the chemotherapy is rendered harmless by attachment of another bioorthogonal chemical. And it floats throughout the body, throughout circulation, doing nothing. It’s totally harmless. But when it encounters that material that was injected on the previous day, the two bioorthogonal chemicals see each other and they react. And that reaction releases the active chemotherapy right there locally in the environment of the tumor.
So you get this burst of toxic drug only in the tumor and nowhere else in the body. And it kills the tumor without these toxic side effects. And they are now performing this procedure on patients in what’s called a phase I clinical trial. So they’re looking to make sure that it’s safe and to figure out what are the right doses of the two components. And if everything goes well, they’ll start a next phase, where they look for reduction of the tumor burden and benefit to the patient.
IRA FLATOW: Yes. So this is for solid tumors then so far?
CAROLYN BERTOZZI: That’s right. Soft tissue sarcoma is an example of a solid tumor that is right now very difficult to treat. There are not good medicines for that type of cancer. And this could be a real breakthrough.
IRA FLATOW: Well, are there other drug applications or other diseases, tumors, you name it, that this might work with?
CAROLYN BERTOZZI: Yes, there’s another group in New York that is gearing up for a clinical trial to target radioisotopes to cancers. And these are like nuclear particles that release radiation that’s damaging to the tumor. Also, it’s a way of basically delivering the radiation very specifically to the tumor and keeping the damage away from other tissues. And it’s kind of a similar strategy.
They put one molecule in first. And it’s actually an antibody that’s armed with a bioorthogonal chemical. And that antibody is like a heat-seeking missile, which goes to the tumor and latches onto it. And then in a second step, they add the radioactive particle which has the other bioorthogonal partner on it. And it will then find that antibody and do the reaction. And now that radioactive particle is also concentrated in the tumor.
So it’s kind of a similar approach. But instead of a chemotherapeutic drug, it’s a radioactive particle. And instead of a polymer that gets injected, it’s an antibody that finds its way. But I think that theme is one that you’ll find being tested again and again and again. And it’s something you really couldn’t even conceive of doing without the availability of bioorthogonal chemistries.
IRA FLATOW: Yeah. You talk about a heat-seeking missile. Back in the day, we used to call it a silver bullet.
CAROLYN BERTOZZI: Yeah, that’s right. And Paul Ehrlich, right, the magic bullet.
IRA FLATOW: Yeah, that’s right.
CAROLYN BERTOZZI: That’s a 100-year-old concept. And finally it’s having a huge impact in the way that we design medicines.
IRA FLATOW: This is Science Friday, from WNYC Studios.
In case you’re just joining us, I’m talking with Nobel Prize Laureate Carolyn Bertozzi about chemistry in living cells.
I’ve heard the work your co-Nobelists did, called click chemistry. Is this kind of a click chemistry you’re talking about?
CAROLYN BERTOZZI: Yes. There are many overlapping concepts between bioorthogonal and click chemistry. And that’s why I think the Nobel Foundation put us together into this one Prize. The “click chemistry” term was coined by Barry Sharpless. And he shares the Prize with myself and another chemist named Morten Meldal.
Barry was interested in reactions that have the property that they’re very reliable, they form products in very high yield, and they do so without interference by other functional groups on the same molecules. So you can see how that’s a similar challenge to our own thinking about bioorthogonal chemistry. The big difference is that when Barry was conceiving of click chemistries, he was thinking about them as tools for the synthesis of complex molecules. Which is another big challenge in the chemical sciences.
And Morton Meldal had a similar motivation. Both he and Barry are synthetic chemists. They like to make big complicated molecules. And having these click chemistries can get you to those big complicated molecules much more easily.
For bioorthogonal chemistry, there is a separate challenge. Which is you want the chemistry to be able to go in live cells, live animals, live human beings. So there are some other challenges that we had to overcome that are not so relevant when you’re synthesizing complicated molecules with click chemistry. But there definitely are overlapping concepts.
And so when we invented a bioorthogonal chemistry that was based on some of the same chemistries that Morten and Barry were developing, we took one of our bioorthogonal chemistries and we branded it copper-free click chemistry because it was similar to one of their click chemistries that had a copper catalyst in it. So we borrowed on their branding. But the bioorthogonal mandate has some different challenges that you don’t have to worry about if you’re making molecules in the reaction flask, in the chemistry lab, and not in the human being.
IRA FLATOW: So let’s talk about extending your work out further. What would you want your work, your chemicals, to be able to do that they can’t do now, or you can do with them?
CAROLYN BERTOZZI: Well, that’s a great question. Right now, the only bioorthogonal chemistry that has risen to the level where you could think about doing it inside humans is a reaction called the tetrazine ligation. And that is the chemistry that’s being used right now in these clinical studies. And that’s a great chemistry, but it would be nice to have an expanded toolbox of chemistries that you could do in humans. We need more than one.
And there are other bioorthogonal reactions that we and others developed and have used in animals, in cells in a dish, in lots of other settings. But to actually do the chemistry in the human body is a whole other layer. And right now we only have one chemistry that is really good enough for that.
So I think inventing new chemistries that really push the envelope, that you could do in people, is something that’s still an open challenge. And then there are applications beyond these clinical applications for cancer drug targeting. Molecular imaging in humans is still a challenge that has yet to be reached, and we continue to work on that. And then there are lots of applications outside of biomedicine– for example, in material science– and I think the iceberg’s tip is just being explored in that area as well.
IRA FLATOW: We need to take a break. And when we come back, our conversation with Nobel Laureate chemist Carolyn Bertozzi continues. Stay with us.
This is Science Friday. I’m Ira Flatow. We’re talking this hour with Dr. Carolyn Bertozzi, 2022 Nobel Prize Laureate and professor of chemistry at Stanford University, in Palo Alto, California.
I’d like to broaden our conversation a bit from your specialty work to chemistry in general. People say chemistry is so boring, right?
CAROLYN BERTOZZI: People are wrong.
[LAUGHTER]
IRA FLATOW: Well, this is a great example of that. What sort of advice would you give people coming to you, for future scientists? I’m sure you would point them in your direction. Because as we say, chemistry is a lot less boring than you think it is.
CAROLYN BERTOZZI: That’s correct. I think the misconception of chemistry being boring might be our own fault. Because I think chemists teach students, and usually the first exposure is somewhere in high school. I think we teach those high school students in a kind of boring way.
IRA FLATOW: You think?
CAROLYN BERTOZZI: I think so.
IRA FLATOW: As someone who took chemistry in high school, I can tell you that’s the truth.
CAROLYN BERTOZZI: It was true for me, too, I hate to say. I took chemistry in high school. And I don’t know that I hated it, but I didn’t like it.
IRA FLATOW: Yeah. Yeah, my worst subject
CAROLYN BERTOZZI: Yeah. It was boring, and I didn’t understand– it didn’t seem relevant to me or the world at all. And then I went to college. And at first I was a pre-med. And so you have to take some chemistry classes if you’re destined for medical school. So I did– not because I wanted to, but because I had to. And even in freshman chemistry, I was not interested. Again, it didn’t seem all that relevant or interesting to me.
So I’m just like you in that regard. And if I had not taken organic chemistry, I think I probably wouldn’t have discovered the field as an exciting field. But organic chemistry turned things around for me. And that’s when I realized that chemistry is so central in biology and biomedicine. And if you want to understand disease, human disease and figure out how to treat diseases, you really need to be a chemist.
So I’m really glad that I stuck with it long enough to discover organic chemistry, because that sealed it for me. But I think if we did a better job teaching in the early stages, people wouldn’t have this bias against chemistry. It’s fascinating. And there’s so much we don’t know and so many discoveries yet to be made, it’s a field I think of as still very young.
IRA FLATOW: Interesting. OK. So now you have this huge stage. As a Nobel winner, you’re in the spotlight. Are there things you want to use that power or visibility to try to do that maybe you couldn’t just as a chemist?
CAROLYN BERTOZZI: This is a really interesting question. And it’s only been a few weeks that I qualify as a Nobel Laureate. So I don’t think I fully understand yet what new power I might have– unbeknownst to me. But even in the last few weeks, I have noticed that people are now paying more attention to things that I say. And I’ve been saying the same things all along, but now, suddenly, they have more gravity, I think, because of my status as a Nobel Laureate. So that’s great. Because I certainly have philosophies that I would like people to think about and maybe even embrace having to do with the diversity of scientists needing some enhancement in order for us to do the best science we can do.
Especially in the physical sciences, like chemistry, there are many different underrepresented groups who have been historically excluded from the field and whose talent we haven’t been able to avail ourselves of. Which limits progress. Hopefully now people will pay attention to the importance of diversifying the chemical workforce so that we can take advantage of all the talent. That’s one thing I would like to convey.
And the interesting thing is lots of chemists, including myself, have been advocating for greater diversity among our ranks. But if you are awarded the Nobel Prize, and your strategy for success has been to have the most diverse lab that you can have, I think that that gives some validation to the idea that diversity breeds success.
IRA FLATOW: That’s a very Nobel and noble thing to pursue.
CAROLYN BERTOZZI: Yeah. Thank you.
IRA FLATOW: I’m not going to let you go yet, because I’ve heard you have a hidden talent outside the lab. And that is music. You were in a band called Board of Education, with Tom Morello, of Rage Against the Machine. How did that happen? And I mean, now, are you as famous as he is?
CAROLYN BERTOZZI: That is correct. First of all, yes, I had the great privilege way back in college, in the mid-1980s, of playing in that band with Tom. It was his band. He was a Harvard student as well, about two years ahead of me. And he recruited me to join the band, and I got to play with him. And we played ’80s pop music in frat parties and so on. But he also had original compositions that I played with him. And it was amazing.
So we discovered each other because we were in college together. And I am not as famous as him.
[LAUGHTER]
And I don’t know that I ever will be. But he was kind enough, after I won the Nobel Prize, to tweet a congratulations. And I think that that tweet brought more attention to me than any other social media engagement I’ve ever had. So thank you, Tom.
IRA FLATOW: Well, I think Morello obviously is a rock star. But I think winning the Nobel Prize must feel at least a little bit like stardom, though.
CAROLYN BERTOZZI: I mean, for a scientist, that’s about as much stardom as you could imagine ever achieving.
IRA FLATOW: Well, I’m going to leave it right there. Great story of your life and of your research. And thank you for taking time to join with us today.
CAROLYN BERTOZZI: Thank you so much for having me.
IRA FLATOW: Dr. Carolyn Bertozzi is professor of chemistry at Stanford University, in Palo Alto, and 2022 winner of the Nobel Prize.
Copyright © 2022 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/.
Jason P. Dinh is Climate Editor at Atmos Magazine in Washington, DC. He previously was an NSF-funded intern at Science Friday.
As Science Friday’s director and senior producer, Charles Bergquist channels the chaos of a live production studio into something sounding like a radio program. Favorite topics include planetary sciences, chemistry, materials, and shiny things with blinking lights.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.