Can A New Vaccine Put An End To Malaria?
16:52 minutes
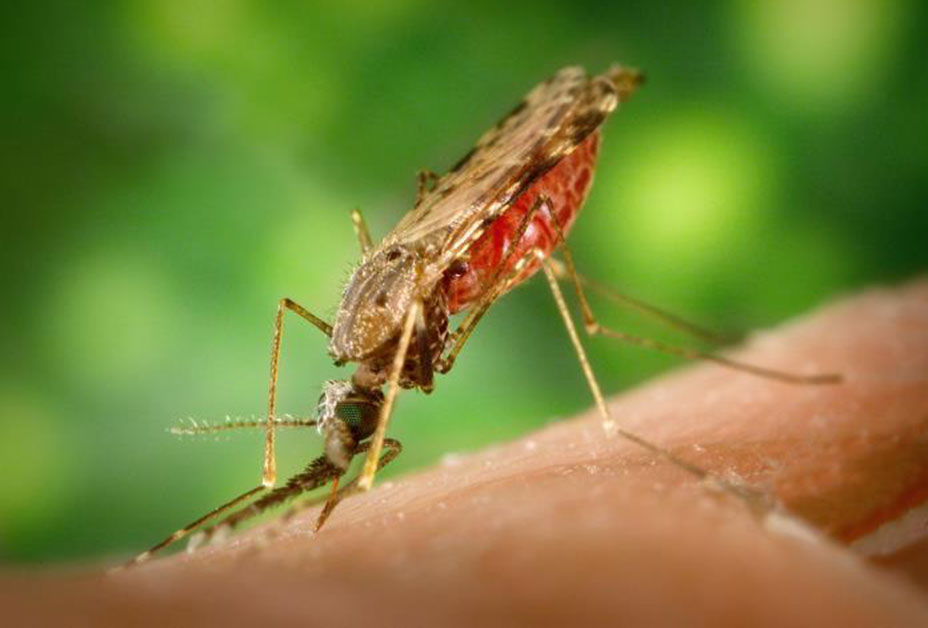
The World Health Organization estimates that every two minutes, a child somewhere in the world dies of malaria. As of 2018, the parasite-induced disease kills a total of more than 400,000 people every year—most of them children under the age of five in sub-Saharan Africa.
While the quest for a malaria vaccine is more than 50 years old, there is still no licensed, fully approved option. The closest to approval, called RTS,S, is being piloted in several countries, with efficacy estimates hovering around 56 percent.
But after a new vaccine, called R21, demonstrated more than 75% efficacy in a small trial in Burkina Faso, is there hope for a more efficient push to reduce the global burden of malaria?
Ira talks to malaria vaccine researcher Prakash Srinivasan and Biden administration malaria coordinator Raj Panjabi about the implications of a vaccine milestone—and the work remaining ahead. Plus, how the COVID-19 pandemic might inform future progress in global health.
Prakash Srinivasan is an assistant professor of Molecular Microbiology & Immunology in the Malaria Research Institute at Johns Hopkins University in Baltimore, Maryland.
Raj Panjabi is coordinator of the U.S. President’s Malaria Initiative at the United States Agency for International Development, founder of Last Mile Health, and a physician based in Washington, D.C..
IRA FLATOW: This is Science Friday. I’m Ira Flatow. Every year, malaria, a disease caused by a bloodborne parasite, kills more than 400,000 people. Most are children under the age of five. The malaria parasite has long been one of the major causes of global deaths, in poor countries, especially. The first and only licensed vaccine so far, called RTS,S has shown only a 50% efficacy rate in clinical trials.
But now, a team of researchers from Oxford University have preliminary trial results for another promising vaccine, called R21. And this time, the efficacy was over 75%. I have two experts to talk about this. First, Dr. Prakash Srinivasan, an Assistant Professor of Molecular Microbiology and Immunology at Johns Hopkins University’s Malaria Research Institute. Welcome to Science Friday.
PRAKASH SRINIVASAN: Thank you, Ira, good to be here.
IRA FLATOW: Nice to have you. OK, let’s talk about this new vaccine, R21. How does it work to arm the body against malaria parasites?
PRAKASH SRINIVASAN: So the parasite has a complex life cycle. And as you rightly pointed out, it’s the forms of the parasite that grows inside the red blood cells, or the erythrocite, that causes disease. When the mosquito feeds on a human host, it takes up these parasites, along with the blood meal. Certain forms of the parasite enter into the salivary glands, or the saliva, of the mosquito such that, when this infected mosquito feeds on another naive vertebrate host– in this case, our humans– it transfers, along with the saliva, the parasites and deposits into the skin of the host.
From there, the parasites find their way through the blood vessels to the liver, specifically. And it is these stages of the parasites that this new R21 vaccine targets. And this parasite stage, because it does not cause any clinical symptoms, is also referred to as the silent stages of the parasite life cycle.
And so the R21 vaccine– the researchers at the Jenner Institute in Oxford, what they have done is to, similar to a spike protein on the coronavirus, they’ve used the protein on the surface of these parasites. And using adjuvants that train our immune system to make antibodies against these proteins, they’ve been able to generate enough antibodies to prevent the parasites from infecting the liver cells. But this R21 vaccine essentially has built up on the success of the RTS,S vaccine, essentially by targeting the same protein but providing it in a form that is making more antibodies against that protein and therefore more effective than RTS,S in these preliminary stage trials.
IRA FLATOW: Is that effectiveness, that 77% number, would that be good enough to release to the world?
PRAKASH SRINIVASAN: Well, a measles virus vaccine is over 90% effective. And we’ve heard about the SARS-CoV-2 vaccines that are over 90% effective. And so to be really effective, we want as close to 100% as possible. But 77% is definitely a big improvement over current RTS,S vaccine efficacy.
But I should point out, however, that even at the early stages of the trials for RTS,S vaccine done in different countries around the world, efficacy ranged anywhere from 50% to even 80%. So it remains to be seen, when R21 vaccine is tested in different conditions, different transmission rates, in different countries, what the efficacy of the vaccine is going to be. But it’s something to look forward to for the next stages of the clinical trials.
IRA FLATOW: What happens in the next stages? And what happens to the actual approval process?
PRAKASH SRINIVASAN: Yeah, so taking a vaccine candidate from discovery at the bench to widespread deployment is a very complex, lengthy, and expensive process. And so the stage where the R21 vaccine is often called the phase II study, where it is done in a few individuals. In this case, the group size was about 150 kids ranging from five months to 17-months-old.
And in the next step, which is called the phase III, that would be expanded to thousands of individuals and across multiple sites. And that is going to evaluate both the safety, efficacy, and immunogenicity under different conditions. Then the next step would be for it to be taking a positive opinion from the WHO and towards licensure.
IRA FLATOW: Do you see the R21 as becoming the sole vaccine? Or do you think the other one is still useful in the long run?
PRAKASH SRINIVASAN: Both the vaccines target the same protein on the parasite surface. So if one is better than the other, certainly my view is that one would be sufficient. But your question points to a much bigger problem, which is, even if they are able to achieve 77% efficacy consistently, that still means 23% of the people who would go on to develop an infection.
And since this vaccine targets the silent stages of the parasite, clinical disease is still possible in those individuals the vaccine was not effective. And therefore, an eventual malaria vaccine, I suspect, will be also targeting the forms of the parasite that causes disease. That is the parasites that grow within the red blood cells.
And so, for example, in our lab, we are focusing on a parasite protein where the parasite injects this protein onto the host cell’s surface to enter into their host cells. So we think that targeting such important interactions could be a way forward for developing an effective blood stage malaria vaccine and that eventually combining vaccines that target such blood stage parasites, along with vaccines like RTS,S and R21, to perform the multi-stage, multi-target vaccine is going to be more effective in the long term.
IRA FLATOW: Now, we know that researchers have been working on a malaria vaccine, what, since the late 1960s. Can you tell us why there is a reason that malaria has been so difficult to develop a vaccine against?
PRAKASH SRINIVASAN: So unlike some of the viral vaccines, the viral genome codes for a few proteins. The malaria parasite genome codes for over 5,000 proteins.
IRA FLATOW: Wow.
PRAKASH SRINIVASAN: Which protein do you target? Which candidate do you target? The parasite also changes its code to escape the immune system. And so a vaccine, for it to be effective, has to make sure that the targeting protein are effective, that the antibodies targeting those proteins can effectively prevent parasites from causing disease or infection but, at the same time, also allow for preventing the mutations from happening. And if it does happen, that you have a backup strategy to prevent those parasites from infecting.
The second important thing is the life cycle of the parasite itself. As I mentioned to you, the parasite’s life cycle critically depends on the mosquito vector. While that might be thought of as a bottleneck, that is also a step that has eluded parasites from being effectively cleared from the immune system because there’s always the mosquitoes carrying the parasites and it’s much more difficult to eliminate all the mosquitoes, as you probably are aware of in your own backyard, to get rid of all these mosquitoes during the rainy season.
IRA FLATOW: Is there enough money to look at all these different ideas?
PRAKASH SRINIVASAN: Well, I would say certainly, the funding for malaria research is not where it should be. And I suspect the challenge comes from the fact that most of the countries that are directly affected by malaria are developing countries. And therefore, we do need a lot more investment in malaria.
So there’s been a lot of work on characterizing different vaccine candidates. And as I mentioned to you, there is a number of steps along this pathway towards vaccine development. But as soon as the preclinical studies are done in the lab, progressing candidates to clinical trials is a big hurdle, because there’s a substantial requirement of investment to move learnings from the lab into the field. And to be able to test it in clinical trials requires a lot of funding of investment.
IRA FLATOW: Well, thank you for keeping us up to date on this.
PRAKASH SRINIVASAN: Absolutely, it was a pleasure to speak with you, Ira. Take care.
IRA FLATOW: Dr. Prakash Srinivasan, Assistant Professor of Molecular Microbiology and Immunology, Johns Hopkins University’s Malaria Research Institute. I want to turn now to the bigger picture of eliminating malaria worldwide. This is a goal the World Health Organization is working toward with help from partners around the world, including, in the US, an office called the President’s Malaria Initiative, with a budget of $770 million per year. The president’s Malaria Coordinator joins us, Dr. Raj Panjabi, a Physician and Founder of the nonprofit Last Mile Health. Welcome to Science Friday.
RAJ PANJABI: Thanks for having me on, Ira.
IRA FLATOW: It’s our pleasure. You know, we just heard a lot about the decades-long effort to develop vaccines against malaria. And there’s still nothing fully licensed or available in the world yet. Where could more resources make a critical difference?
RAJ PANJABI: First of all, as far along as the current vaccine candidates– are and it’s very exciting to see– we do need more investment in developing more tools, including more candidate vaccines. We also need investment in the tools that we know work– medicines that save lives, tests that allow you to detect malaria, and bed nets, and mosquito sprays that actually kill the mosquitoes that carry malaria to humans. So those are the areas where we need further investment.
But we also need it in health workers, nurses, and community health workers that are on the front lines of delivering treatments for, and tests, and sprays, and bed nets to families around the world. And we’ve got to do more to invest in them.
IRA FLATOW: The World Health Organization has set this goal of reducing malaria mortality by 90% by 2030. That’s almost around the corner. How much could vaccines help?
RAJ PANJABI: It’s worth taking stock of the progress we’ve made so far. I grew up in Liberia, which has one of the highest burdens of malaria in the world. When I was first infected with malaria as an infant in 1981, nearly a million people died that year from the disease. By 2004, that number had nearly doubled to about 2 million.
But since then, the world has made incredible progress. A billion and a half infections have been prevented since 2000. Dozens of countries are moving towards elimination. So we’ve also seen, in the countries we directly work with, death rates amongst children have fallen by about 60%. So there’s a lot of cause for optimism.
But as you said, to reach that goal of reducing the disease and the death rate further and also moving towards ending malaria within our generation, we’ve really got to face these major threats. COVID has set us back. Testing rates in parts of the world for malaria have fallen by 31%. And the WHO predicts there could be as many as 100,000 excess malaria deaths, and that’s on top of the fact that more people died from malaria in Africa last year during the COVID pandemic than from COVID-19 itself.
So we’ve got to do a lot more to get back on track to ending malaria if we’re going to have a chance of doing it within our generation. And I still think that’s possible. But we’ve really got to rethink the way we deliver the tools we have and also develop new tools. If we want to move the needle from 400,000 deaths a year down towards zero, we’re going to have to reach the unreached– so in these areas where you might have to walk, hike through a forest to reach a village where there’s no clinic or no physician for hours or days, in some cases.
IRA FLATOW: This is Science Friday from WNYC Studios. You know, I’m listening to this, and I’m almost matching it one-for-one the words I hear from COVID-19– trying to reach people who are unreachable. How similar is that?
RAJ PANJABI: You know, there is a truism in infectious disease that the poor and the marginalized are more likely to be left out of reach of new medicines, new vaccines, new tests. A billion people are still out of reach of the most basic of medical advances. About 13 million children every year don’t even get a single dose of any vaccine. That’s for measles or for other conditions.
There are models of care that allow you to deliver care, to go that extra mile, and reach those that have been unreached. A lot of it starts by shifting the way we think in medicine from bringing care to patients rather than waiting for patients to come to care, meeting people where they are.
And if you look, Ira, at the most successful vaccination programs, the ones that succeed in reaching marginalized communities, whether it’s in rural areas or poor urban areas, they often have a community-based model. They bring vaccination sites from hospitals into community centers, sometimes all the way to the doorstep. Those are the types of things that we need to do if we’re going to succeed in bending the curve and defeating these pandemics.
IRA FLATOW: Well, it sounds like what you’re saying is that we need an ongoing system. I mean, we’re jumping from one disease to another, one pandemic to another, and then setting up these systems on the fly almost. It’s like these systems don’t stay around so that they can remain and be applied to the next disease.
RAJ PANJABI: I think you’re right. The most effective response– it may not be an emergency response system, per se, but an everyday system that’s able to surge and respond to an emergency when it happens– a few years ago there was an Ebola epidemic in West Africa, as we all remember. And that took many thousands of people in Liberia, many hundreds of health workers. The reason that spread so widely is because, in rural areas of the country, clinics and lab systems and health workers were too few. And we just didn’t see it until it was already widespread.
After the Ebola epidemic the US government, with others, worked with the Liberian government to do a number of things. One of the most important investments they made was to hire, train, and equip local health workers, community health workers, outreach nurses to reach those rural areas that had not been reached before, where the highest risk of future epidemics are likely to emerge. Those workers first we’re looking for the next Ebola. But they were also leveraged by us at PMI to deliver testing and treatment for malaria.
So all of a sudden, in a country where malaria is the ranking problem in the country– about 50% are sick with malaria at any one point– we actually were able to extend the reach of malaria testing and treatment, because these community health workers went door to door. Now, when COVID-19 hit, it’s actually these same workers that are going door to door to screen patients for COVID symptoms.
IRA FLATOW: This past year has certainly shown us how quickly researchers can create a vaccine for a new disease if money and the collective energy is there. Do you think we’d be able to get sort of Operation Warp Speed for malaria vaccines to help us finish what has already been started?
RAJ PANJABI: Let’s first acknowledge we’ve been not only tracking but investing in malaria vaccine development for many, many years– in fact, for more than 50 years. And since 2000, we’ve invested $130 million. This has been 100 years nearly in the making since the parasite of malaria was first discovered.
So one of the critical issues is certainly the political and economic attention. The other is the complexity of malaria. And so what we need, in addition to seeing progress through on these latest vaccine candidates, is to ensure we’re doing much more to invest in upstream or early-stage research. That has all kinds of benefits, Ira, not only for helping us accelerate progress on making malaria vaccines, but also in expanding knowledge for vaccine delivery systems for other pathogens– in fact, for COVID-19 itself. And so while we need to do more on COVID vaccine research, the more we do on malaria vaccine research, not only will that be a win for children and families across the world that suffer with this disease, but it actually can help us advance science against other conditions, as well.
IRA FLATOW: Well, this has all been very interesting. I want to thank you for taking time to be with us today.
RAJ PANJABI: Thank you, Ira.
IRA FLATOW: Dr. Raj Panjabi, Coordinator of the US President’s Malaria Initiative.
Copyright © 2021 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Christie Taylor was a producer for Science Friday. Her days involved diligent research, too many phone calls for an introvert, and asking scientists if they have any audio of that narwhal heartbeat.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.