Is A New HIV-Prevention Drug Worth The Extra Cost?
5:04 minutes
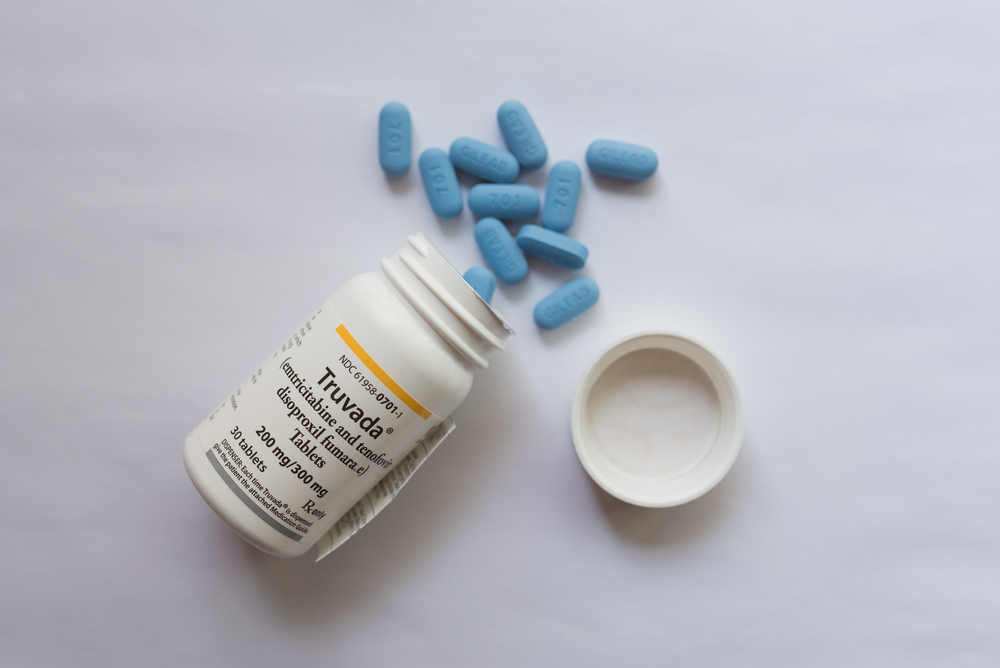
In 2012, the FDA approved the drug Truvada, the brand-name HIV pre-exposure prophylaxis (PrEP) that HIV negative people can take to prevent contracting the virus. The patent for Truvada is due to expire, which would allow for more generic versions of the drug. But Gilead, the manufacturer of Truvada, is releasing a second brand name PrEP called Descovy.
Physician Rochelle Walensky, who is chief of the infectious disease division at Massachusetts General Hospital, is an author on a study in the Annals of Internal Medicine that weighed the financial and accessibility impact that this new drug will have for patients.
Rochelle Walensky is a Professor of Medicine at Harvard Medical School and Chief of Infectious Diseases at Massachusetts General Hospital in Boston, Massachusetts.
Alexa Lim was a senior producer for Science Friday. Her favorite stories involve space, sound, and strange animal discoveries.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.