Minimalist Biology: Craig Venter’s Latest Life Form
17:29 minutes
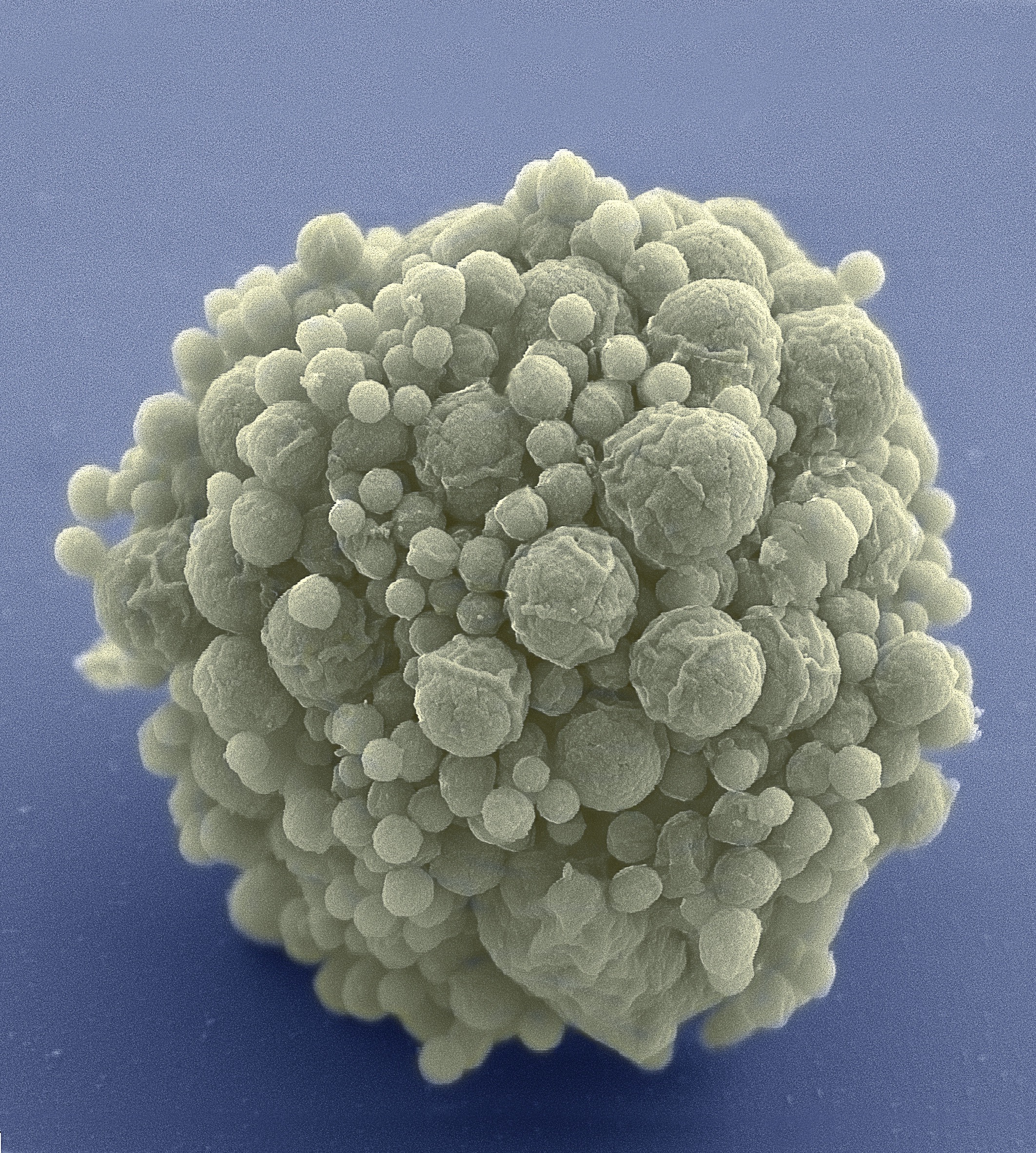
Less is more for biologist Craig Venter and his team, who have booted up a cell with only the bare minimum genetic instructions required for life, encoded in synthetic DNA. After years of failure, they discovered that 473 genes are all that’s needed to create a living, stripped-down version of the bacterium Mycoplasma mycoides.
Despite the fact that Venter and his team still don’t know the function of a third of the organism’s genes, they said at a press conference this week that synthetic life could serve as “a very useful chassis for many industrial applications, from medicine to biochemicals, biofuels, nutrition and agriculture.” They detail the findings in the journal Science.
J. Craig Venter is author of Life at the Speed of Light: From the Double Helix to the Dawn of Digital Life (Viking, 2013); founder, chairman and CEO of the J. Craig Venter Institute; and founder and CEO of Synthetic Genomics Inc. in La Jolla, California.
IRA FLATOW: This is Science Friday. I am Ira Flatow. You’ve probably heard of minimal art, minimal music, minimalist design and architecture. How about minimalist biology? If such a thing exists, then my next guest, Dr. Craig Venter is at the avant garde. You may remember a few years ago, he and his team synthesized a complete genome from scratch, and booted up a bacterial cell with it. Now, they’ve taken a second look at the synthetic genome, snipping and slicing it down to the very bare minimum number of genes needed to bring a cell to life. How many is that? I’m not going to spoil the surprise, let’s have Doctor Venter here tell us himself. Craig Venter is a biologist and a founder and CEO of the J. Craig Venter Institute in La Jolla. Welcome back to Science Friday.
DR. CRAIG VENTER: Thank you, Ira. It’s so nice to be with you again.
IRA FLATOW: Thank you. So what’s the answer? How many genes, really, are essential for life in your study?
DR. CRAIG VENTER: So, essential is a context-specific question. So the number would not be an essential number for humans, or even an essential number for surviving in the wild, but surviving in our laboratory conditions with well-grown things, the number comes out at only 473 genes.
IRA FLATOW: Wow.
DR. CRAIG VENTER: But I think the biggest surprise is 149 of those are of complete unknown function, and this is a very different view of life than we’ve had for the last 20 years.
IRA FLATOW: Wow, so we really don’t know why it needs 149 of those genes, or what those genes are doing. But it won’t survive without that.
DR. CRAIG VENTER: That’s right. So we know they’re essential for life in this minimal organism and its growth conditions, but it’s interesting. So in 1995, my team sequenced the first two genomes in history, and we and everybody else compared those two to each other, and these studies have been ongoing for the last 20 years, and people decided the number of minimal genes was somewhere between 256 and like, 320 or so.
And we even started to believe that ourselves, and we set out to design a cell from scratch in the computer, assuming this community knowledge of biology was actually necessary and sufficient to define life. And we designed a cell with– it was a little bit more than 256 genes. And we had a contest, and we had four or five different designs. We tried them all, and they all failed. So life by design, a pure, first principled design, did not work.
And so what we had to do, we knew that when five years ago, when we built our Syn 1.0 genome, that we had constructed all those genes correctly, because we had this living cell. So we took sets of genes from Syn 1.0, and then added it back to our design set, and we kept adding back until we could get a living cell. And then we called that 2.0, and then we worked to work out which one of those were really not necessary. And it’s not so easy as just pulling one out and testing it.
So, if you were new to this planet, and you’re looking at a 737, and you’re trying to work out its function by pulling parts off.
IRA FLATOW: You mean the airplane, the 737.
DR. CRAIG VENTER: Yes. And you found these pod-like things hanging off the wings, and you removed the one from the right wing, you would find that the airplane could still fly and land, you would say, obviously that part is not necessary for airplane flight. And so, if you then applied that rule, and you say, well, there’s one on the other wing as well, we can obviously dispense with that. And you throw that out and all of a sudden, the airplane crashes.
So that’s what happens in biology, is we have pairs, or more than pairs of things, that either have similar functions, or coexistent functions that you have to have both parts there. And then it turns out 32% of these parts are unknown. So, we’re truly like the aliens from another planet trying to examine the airplane and work out what its parts do.
IRA FLATOW: Is this frustrating to know that you don’t know what those 149 genes do?
DR. CRAIG VENTER: Well, it’s frustrating, but it’s also enlightening, because the scientific community has been basically suffering from the delusion that we did know all of biology. And so, the fact that we’ve been missing a third or so is– it’s exciting, because we can now move and try to understand it. You’ve been covering science forever, and so you know it’s one of the ironies of science, is you can only study the known world.
Our entire view is based on what we know currently as reality. Well, it turns out we were missing about a third of reality in terms of biology. So that’s probably the most important finding, and it’s frustrating, it’s humbling, it’s exciting. It’s all of the above.
IRA FLATOW: Tell me what the point from just purely, like Lincoln Logs or LEGOs, putting together the minimum amount of genes you need for a living organism, what use would you make out of this, now that you can do this?
DR. CRAIG VENTER: Well, proving that we can do it, and this is the first time that somebody, to my knowledge, has attempted to actually design a species in the computer and then bring it to life. It tells us about the fundamentals of biology. We probably never would have discovered this third of essential genes without this experiment, and so really trying to get down to the essence of life, to define it in this complex genomics software fashion, tells us maybe more about how they came together. It really tells us that we need to look at life in a genome-centric view, versus what’s been done for a long time of a gene-centric view. We can’t just look at a function of a gene, we have to look at it in the context of a whole.
Like an orchestra, you can take out one violin, or one piccolo, and not substantially change things. But our cells work together in an orchestrated function of having all the components working together. So, I think it really helps us understand a life. It also helps us with the next stage, if we’re really going to do life by design for an industrial revolution of changing manufacturing from agricultural processes to truly cellular manufacturing– an example, for food.
We need to really know what all the components do, and then how to add new components to get where we want to go. So getting to a baseline chassis, just sort of an intellectual point– if we now add components to it, that we understand, we should be able to understand huge steps in evolution. We should be able to cover a billion years of evolution in a year or so, getting to this next stages.
IRA FLATOW: So do have a plan? How do you tease out, then, the genes that aren’t doing anything? I think we used to call it junk DNA back in the day. Do you have a plan to figure out what these genes do? Is there a way to find out why you need them?
DR. CRAIG VENTER: Well, it is a great question, and it’s also an irony of science that we only study the known world. It’s probably impossible to get a grant from the US government to study genes of unknown function, even if they’re essential for life, because it’s hard to put a grant together when you don’t know what they do. So what we’re hoping will happen– well, number one, we’re funding a lot of internal grants at the Venter Institute to try and see if we can decipher some of those. And we’re making some headway.
So, if we’re looking at the gene structure, we can tell, for example, that a protein would be a transmembrane protein, and have all the structural lookalikes of being a transporter of some substance. What we don’t know is what does it actually transport, and why is what it transports essential for life of the cell? So knowing the transporter, then you can design experiments, sort of a trial and error things by eliminating things from the media, or just other mechanisms to see what that transports. Those are probably going to be the easier ones to do.
The ones that are going to be tougher, where the structures don’t look like anything we’ve seen before. So there’s no hints from a structural association. And we don’t know what they do, because we assumed we already had all the essential components in the cell. So maybe they do subtle things of modifying protein, manufacturing or chromosome replication or something. So it’s going to take more effort.
But what we hope happens, because there’s so much research going on globally, it’s highly likely in some lab, somewhere around the world, somebody is studying these proteins, and all of a sudden they’re going to find, when they get to the gene, that we can make a link by homology to one of the things we’re discovering. So we hope to excite the world scientific community to take on this challenge of understanding this important gene set. Because there has to be very exciting new universal biology, because I’d say over half of these genes are highly conserved throughout evolution. They won’t tell us this about our minimal cell, they’ll tell us something about all of life.
IRA FLATOW: Yeah, if they weren’t important, why wouldn’t they be conserved? And since you came out with your first synthetic life, the gene editing technique called CRISPR has really exploded. How does CRISPR fit in? How is it similar and different than what you’re doing? Does it does eliminate the need to build entire genomes from scratch, so to speak, as you’re doing?
DR. CRAIG VENTER: So CRISPRs are a really fantastic editing tool, and we use them all the time in the lab, and Synthetic Genomics is using them all the time. I think you know we have this project at [INAUDIBLE] where we’re trying to edit the pig genome to allow organ transplantation, such as hearts and lungs and livers, between pigs and humans by changing the pig genes to human genes so they’re not rejected. So in some cases, there’s minor differences between the pig sequence and the human sequence. We use CRISPRs for making those edits, and it’s really fantastic how easy that is to do now. I think it’s one of the most wonderful discoveries in recent science.
But other areas, we’re writing entirely new code to put in, so we’re using both approaches. So think of the difference between editing and writing something new. So you could take our science paper and certainly edited it. In fact, that’s what the editors at Science did, they did a lot of editing on it. But there’s no way by editing that manuscript, you would turn that into an exciting book about philosophy.
IRA FLATOW: It’s different, is what you’re saying.
DR. CRAIG VENTER: Yes. Editing is editing, versus de novo creation. So that’s why we value creation so much in works of art, literature, science writing, et cetera. Many people make their living by doing editing, which is very different than de novo creation.
IRA FLATOW: Now, let me remind everybody that I’m Ira Flatow, this is Science Friday from PRI, Public Radio International. Talking with Craig Venter, founder and CEO of the J. Craig Venter Institute in La Jolla.
You know, the first thing people are going to want to know, probably I don’t have to ask, you’ve been asked at press conferences and things like that. Someday, could you design a minimal human genome?
DR. CRAIG VENTER: You know, it’s an interesting question. In fact, somebody recently– I don’t know where the paper was, I just saw the headlines– claim that they had a set of 3,000 minimal essential human genes. And I kind of laughed at myself, because it’s sort of starting where we were 20 years ago with the minimal bacterial cell, and missing most of the metaphors– for example, looking at the airplane.
But you know, all definitions of genomes have to be in context. If we would want those humans to be highly adaptable, to live in all the different environments that we live in, have intellectual adaptation, et cetera, I doubt that we will ever want to do that, even hypothetically. Can people live without some genes? Yes, but that’s what we’re finding at my main enterprise.
Now, human longevity, where we’ve thus far sequenced 25,000 human genomes, we find that people can live without certain genes. But in many cases just mutating those genes causes unusual traits and/or disease. So, more genes– when you look at E. coli, E. coli has almost 5,000-something genes, versus the 400 in this minimal set. But E. coli can survive in lots of different environments, to all kinds of complex metabolism, it can manufacture its own amino acids, versus we provide all these to this minimal cell. So, life is very much contextual, and our software for life has to be defined in terms of that context.
IRA FLATOW: I only have about a minute left, Dr. Venter. What’s the next step here for you, in this project?
DR. CRAIG VENTER: Well, there’s two different directions. So the next steps are, as we talked about, trying to define what these new unknown genes are, and why they’re so essential for life. On the other hand, we’ve developed an incredible set of tools we had to make thousands and thousands of synthetic constructs to do this trial and error thing to find these 149 genes. And now writing the genetic code is 10 times faster than it was five years ago when we made the first synthetic cell.
In fact, Synthetic Genomics sells a robot for automatically assembling DNA. So the field is moving very fast to enable other people to have tools for writing DNA, and doing all kinds of new constructs that we can try and get cells to take over manufacturing of pharmaceuticals, food, chemicals, even construction materials for Mars.
IRA FLATOW: Wow. Sounds exciting. We’ll have to have you back, Dr. Venter, to talk more about this.
DR. CRAIG VENTER: All right. It’s always nice talking with you. Thank you for having me on.
IRA FLATOW: Craig Venter is a biologist and founder and CEO of the J. Craig Venter Institute in La Jolla.
Copyright © 2016 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of ScienceFriday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies.
Christopher Intagliata was Science Friday’s senior producer. He once served as a prop in an optical illusion and speaks passable Ira Flatowese.