Bringing A ‘Ghost Heart’ To Life
20:38 minutes
The human heart is one of the most complicated organs in our body. The heart is, in a way, like a machine—the muscular organ pumping about 2,000 gallons of blood in an adult human every day. But can we construct a heart in the lab? Some scientists are turning to engineering to find ways to preserve that constant lub dub when a heart stops working.
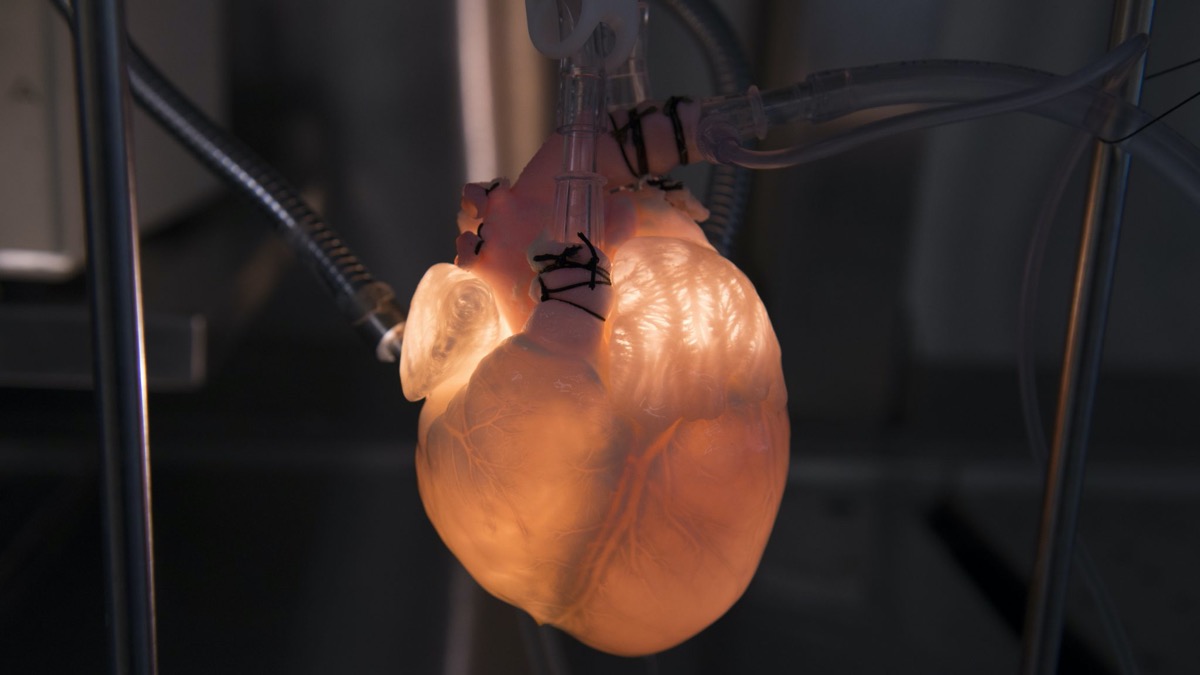
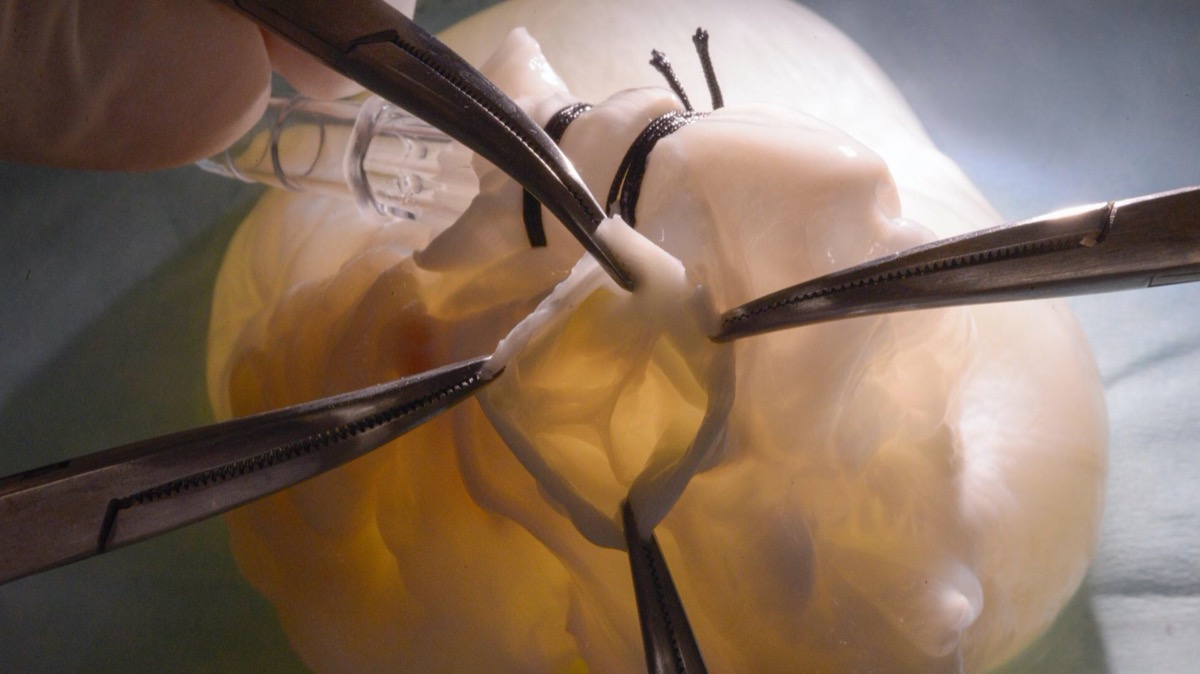
One team of researchers created a biohybrid heart, which combines a pig heart and mechanical parts. The team could control the beating motion of the heart to test prosthetic and artificial valves. Their findings were published in the journal Science Robotics in January. Mechanical engineering student Clara Park, an author on that study, talks about what it takes to engineer a biohybrid heart and how this model could be used in the future to develop implantable hearts and understand heart failure.

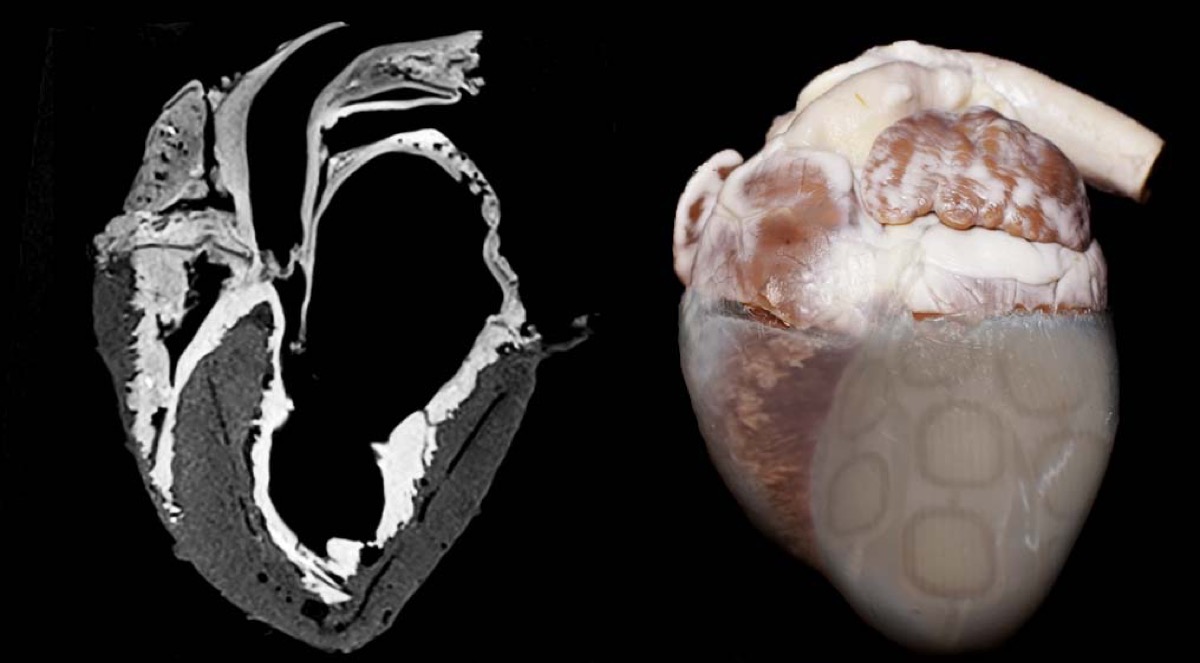
At the Texas Heart Institute, Doris Taylor is developing a regenerative method for heart construction. She pioneered the creation of “ghost hearts”—animal hearts that are stripped of their original cells and injected with stem cells to create a personalized heart. So far, Taylor has only developed the technique with animal hearts, but in the future these ghost hearts could be used as scaffolds to grow transplant hearts for patients. Taylor talks about how much we know about the heart and why it continues to fascinate us.
View more visuals from both labs’ research below.
Warning: The photos and videos below feature images that some viewers may find distressing.
*Editor’s Correction 2/14/2020: A previous version of this page incorrectly stated the name of the journal of the biohybrid heart research. The text has also been changed to clarify that the biohybrid heart model is meant for intracardiac devices such as prosthetic and artificial valves, not pacemakers. We regret the errors.
Further Reading
Invest in quality science journalism by making a donation to Science Friday.
Clara Park is a Phd candidate in Mechanical Engineering at the Massachusetts Institute of Technology in Cambridge, Massachusetts.
Doris Taylor is Director of Regenerative Medicine Research at the Texas Heart Institute in Houston, Texas.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. A bit later in the hour a look at how tech companies are behaving more like countries supplying electricity, internet, and food services that are traditionally administered by national governments. So what happens if they decide it’s no longer in their best interests to do so? We’ll discuss.
But first Valentine’s Day, for those who participate, is designed as a day of romance, an affair of the heart, as the song says. And so for some of you that may well may mean unfortunately a broken heart. I’m sorry if that’s you. But for us, this is an opportunity. What better time than now to explore how scientists are looking at mending human hearts.
And we’re not talking about those bad breakups. Researchers are looking for ways to engineer the heart by building mechanical hearts and creating something called ghost hearts. It’s aimed at making better heart parts, understanding the inner workings of the human heart. And my next guest is part of a team of researchers who hasn’t lost a beat creating a hybrid heart out of a big heart and mechanical parts that can simulate a beating heart outside of the body.
The findings were published in the journal Science Robotics. Clara Park is lead author on that study. A PhD candidate in mechanical engineering at the famous MIT, she joins us via Skype. Welcome to Science Friday.
CLARA PARK: Hi, thanks for having me.
IRA FLATOW: Now, just to be clear, the heart you engineered was a pig heart, correct?
CLARA PARK: Yes, that is correct.
IRA FLATOW: And there were two different systems you needed to build. Tell us about how you designed it.
CLARA PARK: Yes. Sure. So what we did was we had a dead pig heart and we left the inner heart layer intact, but we moved the muscle layers which were no longer functional because it’s outside the body and replaced it with robotic artificial muscles to restore the motion of the beating heart.
IRA FLATOW: So you had basically sort of the skeleton left of the pig heart then.
CLARA PARK: That is correct.
IRA FLATOW: And you put your own what onto that skeleton?
CLARA PARK: So the skeleton part was the inner heart layer. So the reason why we did that was because the heart by its nature has very detailed structures in the heart, like the heart valves and things like that, which are important to its function. So that’s what we kept.
And then the robotic part was where we replaced the dead muscles with the soft robotic muscles to recreate the motion of the beating heart so that we can reanimate it, even outside the body.
IRA FLATOW: Soft robotic muscles. What exactly are they made out of?
CLARA PARK: So soft robotics as opposed to rigid robotics is made out of soft materials. So they are friendly materials like silicone, for instance, that are soft to touch with. And they are good for replicating complex motions like biological motions.
IRA FLATOW: Were you excited? Did it work well? How well did it work in mimicking a live heart?
CLARA PARK: Yeah. So it did work pretty well. So we did make it to be like a real living heart. So it does mimic the pumping motion of the real heart. So I’d say, so usually a healthy physiological heart would pump about 70% of its volume, and ours did achieve to that amount. So we are very happy with that.
IRA FLATOW: All right. Now you have this prosthetic beating heart, what do you use it for? What kinds of useful things can you do with it to learn?
CLARA PARK: So our goal was to use it as a benchtop simulator, as our first goal. So for instance, there are a lot of people with the heart diseases, and one of the common disease is that you can have impaired function of your heart valves. And in that case you’d get prosthetic valves which is designed to mimic a real heart valve to help the blood circulate throughout the body.
And when engineers designed such devices, they need to make sure that there are no leakages or it doesn’t wear away as you have them implanted in your body. So they usually first test them in simple benchtop simulators and then in animals, which can be a very time consuming and expensive process.
So then we realized that there were no machines that could simulate the heart accurately enough for the device designers, so we tried to change that by having a real heart fused with robotic muscles to kind of mimic the realistic motion and anatomy of the heart. So hopefully that could reduce the amount of animal testing in the future or even allow the engineers to iterate the designs more quickly.
IRA FLATOW: So I understand that you’re a mechanical engineering student. How did you start thinking about this from an engineering point of view?
CLARA PARK: So my current training is in mechanical engineering. But my undergraduate degree was also in biological engineering. So I’ve really been at the intersection of the two. And the heart is– I think of it as a both biological and mechanical machine.
So it was a very particularly exciting– and exciting work [INAUDIBLE] engineer. And I think having that dual background kind of allowed me to recognize the limitations and advantages in the two fields and kind of let me combine the two to make a bio-hybrid heart.
IRA FLATOW: Did you ever get frustrated? Did you have a roadblock, you sort of, oh no, how am I going to get past this sort of moment?
CLARA PARK: Yeah. So I think at the beginning when I started this project I think it was a little harder because I had this heart but I didn’t know what to do with it. I didn’t have any medical background and stuff like that. But I was just really started off with dissecting the heart. So I just literally spent a few months doing that.
But in that process I did learn a lot about the heart, and I got really intrigued by the fiber architecture of the heart. So I was just really amazed by how it was organized in such a efficient way to pump the blood, so that actually became the inspiration later on to design the robotic muscles as well.
IRA FLATOW: Did you get a renewed respect for how complicated and intricate the heart works?
CLARA PARK: Yes. Every time I see it, I’m still really amazed by how it works.
IRA FLATOW: Oh, that’s cool. We’re very happy to have you on Science Friday and thank you so much for joining us. And good luck with your PhD work.
CLARA PARK: Thank you so much.
IRA FLATOW: Clara Park is a PhD candidate in mechanical engineering at MIT, and this is an amazing video. We have a video of the heart engineered in action on our website at sciencefriday.com. It’s really kind of cool. Now another way to fix a heart is to grow your own. Yeah. My next guest works with something called ghost hearts. And she’s growing and reviving them in the lab using stem cells in the hopes that one day we can create copies of our own personalized hearts and organs.
Doris Taylor is the director of Regenerative Medicine Research at Texas Heart Institute. Welcome to Science Friday, Dr. Taylor.
DORIS TAYLOR: Thank you. It’s a real pleasure to be here.
IRA FLATOW: What did you think of Clara Park’s work?
DORIS TAYLOR: What a great combination of engineering and biology. You know, that’s what I call bringing a renaissance approach to science. Asking a question and doing whatever it takes to answer it. It’s really cool.
IRA FLATOW: It is cool. Let me give out our number, 844-724-8255 or our audience can tweet us @scifri. I mentioned this thing called a ghost heart. I mean, why call it a ghost heart? What is it?
DORIS TAYLOR: A ghost heart is essentially a heart from which we’ve removed all the cells, leaving the scaffold behind where the cells normally reside. And if you look at it, it looks like a heart and still has all the components of a heart, except the cells. So it looks like a white translucent but opaque heart. And hence the term ghost heart.
IRA FLATOW: And then so OK, you have the ghost heart. It’s white and opaque. What do you fill it up with?
DORIS TAYLOR: So the ghost heart is basically what we call the extracellular matrix protein. And what we do is once we’ve removed all the cells is we transplant stem cells back in. One of the cool things about the heart is that it needs and an inordinate blood supply, an inordinate amount of blood. Our heart beats 24/7, 365.
And every heart muscle cell is surrounded by about four capillaries, four small blood vessels. And so we had to rebuild the blood supply in the heart first. And we did that with vascular cells. And then we transplant back in human induced pluripotent stem cells, stem cells that we’ve differentiated to become immature heart cells.
IRA FLATOW: Now, I’ve seen heart cells growing in Petri dishes where they sort of link up themselves and beat in unison. Is that what happens when you put the stem cells in?
DORIS TAYLOR: You know, they can. They do. They beat. But we actually have to train them. The really remarkable thing about these hearts is that they need a physiology. They need to know they’re in– the cells need to know they’re in the heart, so we have to give them a blood pressure. We have to give them a blood supply. We have to give them oxygen.
And we essentially have to create an artificial body in which these hearts have matured. So we have to pace them until they grow up and learn how to beat together synchronously on their own. And we have to give them a blood pressure to beat against so that they mature.
IRA FLATOW: So they have to think that they’re inside a body somewhere.
DORIS TAYLOR: Absolutely.
IRA FLATOW: Wow.
DORIS TAYLOR: Nature’s pretty smart. And these cells are pretty smart. If we give them the right cues, they do the right things. And the scaffold in and of itself is part of the right cues. Our motto is give nature the tools and get out of the way. We’re never going to figure it all out, but the cells seem to learn what to do when we put them in the right environment.
IRA FLATOW: All right. This is a great time to take a break. And we’re going to come back. That’s great, give nature the tools and get out of– I’m getting out of the way for the break we have to take. Talking with Doris Taylor. Our number, 844-724-8255. So much to talk about with the heart. And you can also tweet us @scifri. We’ll be right back with Doris Taylor after the break. Stay with us.
This is Science Friday. I’m Ira Flatow. We’re talking this hour about engineering the heart. You heard it, the heart. Good Valentine’s Day subject. Doris Taylor, Director of Regenerative Medicine Research at the Texas Heart Institute is my guest. Our number, 844-724-8255. Dr. Taylor, I remember you joined us way back in 2008 when you were first working on ghost hearts in rats. What have you learned in the last 12 years? It’s sort of a stupid question to ask but I’m wondering.
DORIS TAYLOR: You know, 10 years ago, 12 years ago I thought it would simply be a matter of scaling up, taking bigger scaffolds and enough cells, putting them in, and by now we’d be transplanting these in people. Boy was I naive.
However, what we’ve done is move from rat hearts to pig hearts and human hearts. We remove the cells from those routinely now. And as stem cell technology has evolved, and as we’ve– the whole problem and the whole reason we need to grow new hearts is that heart cells don’t divide. They don’t grow by themselves. Or if they do, it’s at a very, very slow low rate.
And so growing the number of cells we need, billions and billions and billions to transplant into a human-sized heart has taken us 10 years. And truthfully, if human induced pluripotent stem cell technology hadn’t evolved, we probably wouldn’t be where we are today.
So I’ve learned how to grow billions of cells [? with ?] my team. And I have to say, this is not me. This is a phenomenal team at the Texas Heart Institute doing this work. We’ve had to learn how to build the artificial body, the bioreactor to put these. We’ve had to build our own pacemakers. We’ve had to build our own artificial lungs, because none of these tools existed 10 years ago.
IRA FLATOW: Do you think– Yeah. I mean, I’m sorry to interrupt, but then do you think it’s beyond our capability when you, it took you 12 years just to figure out how to get all these– to create all these heart cells. It’s beyond our abilities to actually create an artificial but human-celled heart for transplanting.
DORIS TAYLOR: You know, we’ve made more progress in the last year and a half than we have in the last nine. So two years ago, I might have said, wow, we’re close but we’re not that close. And I have said that. But I have the– I have an amazing team. We’ve made huge amounts of progress. And finally the biology, the engineering, the technology, the ability to create the kinds of products you’d just heard about with the bio-hybrid heart means that we’re finally at that tipping point.
I absolutely believe it’s possible. I absolutely believe it’s doable. And I believe within the next two years we will be automating this process in a way that makes it possible for not just my team but a lot of teams to begin to do this.
IRA FLATOW: We go to the phones. 844-724-8255. Brad in Columbia, Missouri. Hi, Brad.
BRAD: Hey, Ira. Glad to be on the air. I was wondering if the researcher could share any thoughts she might have on growing neural tissue and how her work on the heart and scaffolding might impact the ability to regrow spinal nerves.
IRA FLATOW: Good question. Dr. Taylor?
DORIS TAYLOR: You know, the cool thing about the decellularization technology that generate these scaffolds that we use to make the ghost heart is that we can do it with anything that gets a blood supply. Absolutely it can be used to create scaffolds from spinal cord, from brain.
And we and others have decellularized a number of different tissues and organs for exactly that purpose. What is so exciting is that we’re learning that human stem cells respond to their environment in ways that we never anticipated. And I think you’re asking exactly the right question. I think we’re at the beginning of a revolution in regenerative medicine as a result.
IRA FLATOW: We routinely for burn victims and the people who need skin patches, we take skin patches from other parts of the body and we move them around. Can you use your heart structure to create like a patch that we could put into a person’s heart and patch it up?
DORIS TAYLOR: Absolutely. One of the things that we started out doing a few years ago was saying exactly that. OK, let’s put humans– if we don’t have the billions we need to grow a whole heart, let’s take the millions to billion we need to create part of the heart. And we recellularized the ventricle.
And we said, let’s do that and cut it out and use it to make a patch. We and others soon realized that that works. We absolutely can build a patch with the blood supply today. And we can create rings, slices of heart that we put human cells [? and ?] you can watch them beat in the laboratory. It’s phenomenal. It’s amazing. So, yes. But–
IRA FLATOW: There’s another shoe dropping here. I can [INAUDIBLE]
DORIS TAYLOR: Well, no. The other shoe is that every 10 minutes a new person joins the waiting list for an organ.
IRA FLATOW: OK. Let me ask you this question then, because I’m running out of time. Do we need– could you with the sort of Manhattan Project sort of where we say we can do this. We can make lots of heart cells and patches. But you in your little laboratory, Dr. Taylor, we need 1,000 Dr. Taylor’s out there making heart pieces. Then we can move this much more forward.
DORIS TAYLOR: We need that. And we need to automate and close the process. So it’s not about me just hiring more people and more students. And it’s also about resources. The hearts have to eat 24/7, 365. And somebody has to be there right now to feed them.
My team does that. They’re amazing. They never stop. But building the capacity to do it robotically and automating the process, putting enough resources into it, we’d solve this problem. Absolutely.
IRA FLATOW: Now we know the heart is an organ, that there’s a bigger association with it though, because in songs and books the heart is seen as the soul of the body, right? How has your view of the heart changed after working with it so long?
DORIS TAYLOR: You know, it’s humbling. The first time you touch a human heart, at least for me, I cried. And I suspect a lot of surgeons will tell you the first time they held a human heart in their hands, it was humbling. It’s a privilege to do what we do.
It takes a team to do what we do. And I have to say that I’ve also had the privilege of working with artists to talk about the art and science of building a heart. And sometimes there are no words for the privilege we have of doing what we do every day. And if we can make a difference in one person’s life by doing this, then that’s why we are here every day. And thank you for making it accessible.
IRA FLATOW: Well, you’re welcome. I’m getting misty just talking about this with you. And it’s interesting to follow. I know we follow scientists on– over years. We’ve been on almost 30 years and we follow a lot of scientists and where they’re going. So please stay in touch. Would you, Dr. Taylor?
DORIS TAYLOR: Absolutely. Thank you.
IRA FLATOW: Yeah. And it’s a great topic for you to bring us on Valentine’s Day, talking about real heart research. Thank you for your work. And we have all kinds of photos and videos of hearts we’ve talked about in action on our website at sciencefriday.com.
Copyright © 2020 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Alexa Lim was a senior producer for Science Friday. Her favorite stories involve space, sound, and strange animal discoveries.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.