A Cellular Race Through A Maze
16:37 minutes
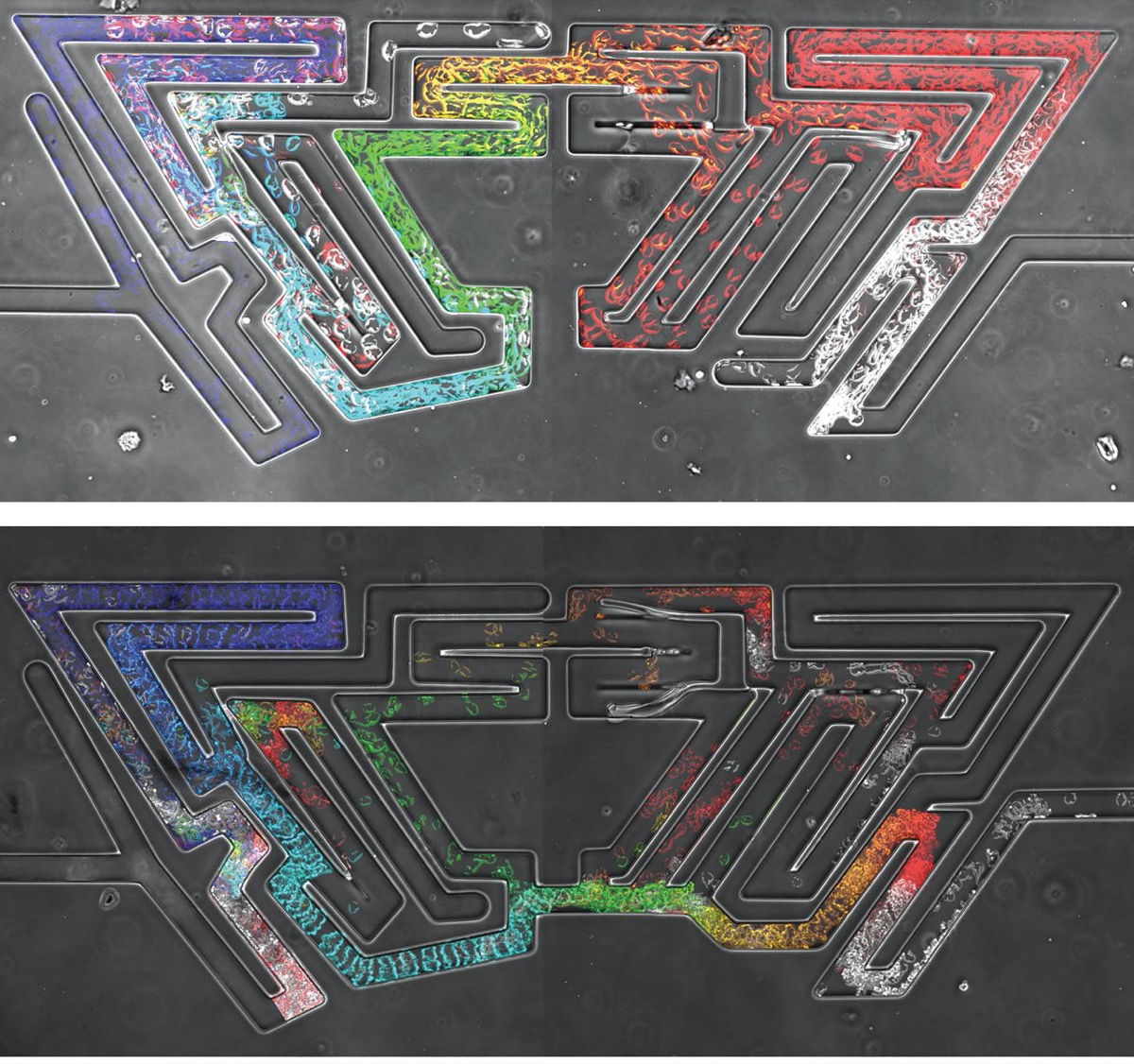
Cells are the basic building blocks of life. Our bodies are made up of trillions and trillions of them, and they all serve a specific purpose. But these tiny workers don’t always stay in the same place. Many move around the body—whether they’re creating a developing embryo, helping the immune system, or, distressingly, spreading cancer.
A team of scientists in the UK recently set up an experiment to learn more about how cells move. They put dirt-dwelling amoebas and mouse cancer cells at the start of a maze, to see how well each would migrate.
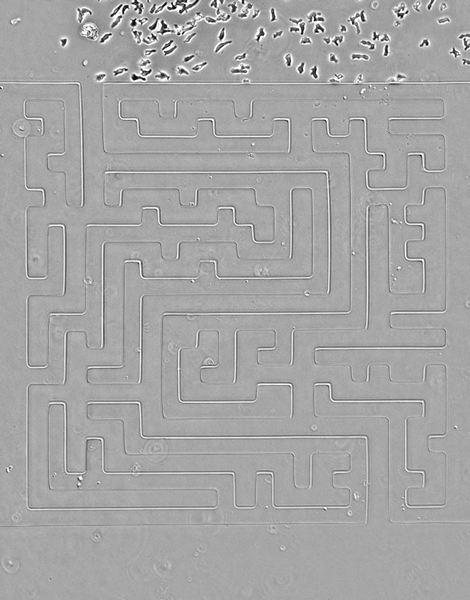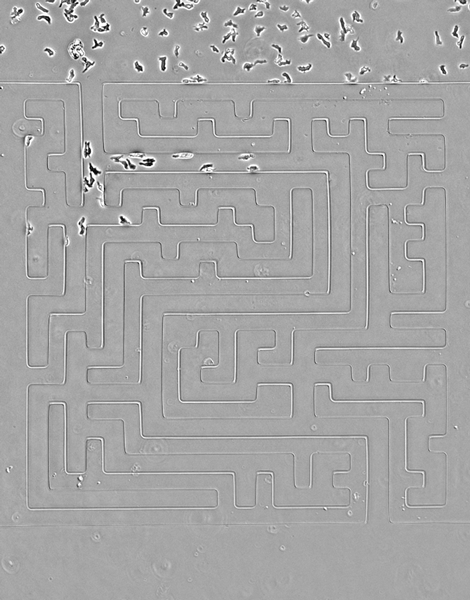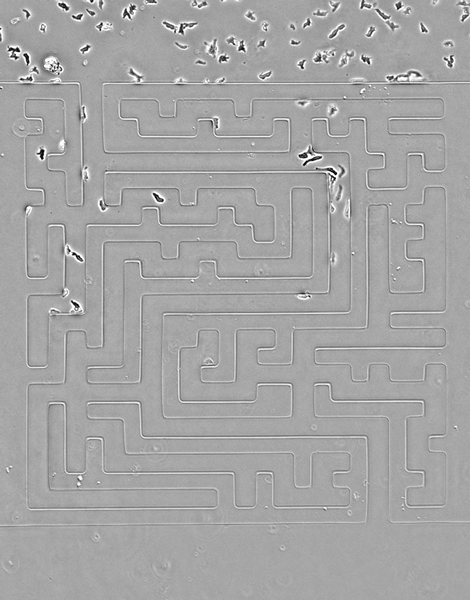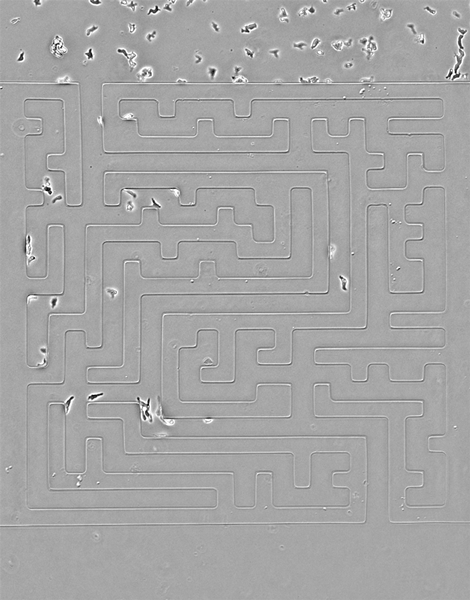
While amoebas proved speedier than their cancerous counterparts, Luke Tweedy, a postdoctoral researcher at the Beatson Institute for Cancer Research in Glasgow, Scotland, says the cancer cells were surprisingly mobile.
“I’m honestly still quite terrified by them,” Tweedy says. “The degree to which they can be guided in one direction is extraordinary.”
Tweedy joins Ira to talk about what his team learned about cancer cell movement, and explains why recreating a famous English hedge maze proved to be a little too difficult for his cellular subjects. See more videos of cells solving mazes below!
Credit: Luke Tweedy, Michele Zagnoni, Cancer Research UK
In the video above, cancer cells (pancreatic cancer—pancreatic ductal adenocarcinoma, grown in culture) solving mazes of different shapes, or a complex maze. They are less perfect than the amoebas, but still scarily accurate, the researchers write.
Credit: Luke Tweedy, Michele Zagnoni, Cancer Research UK
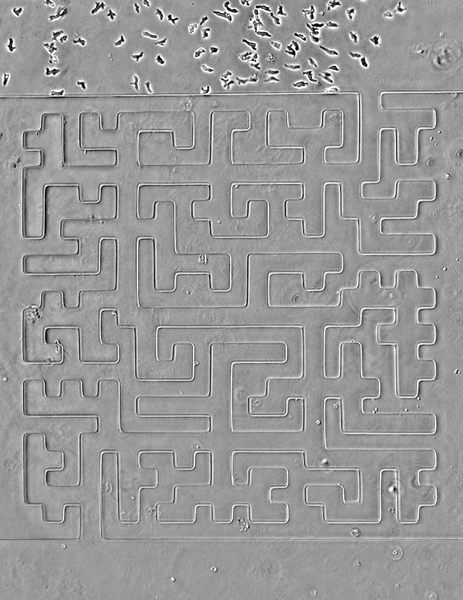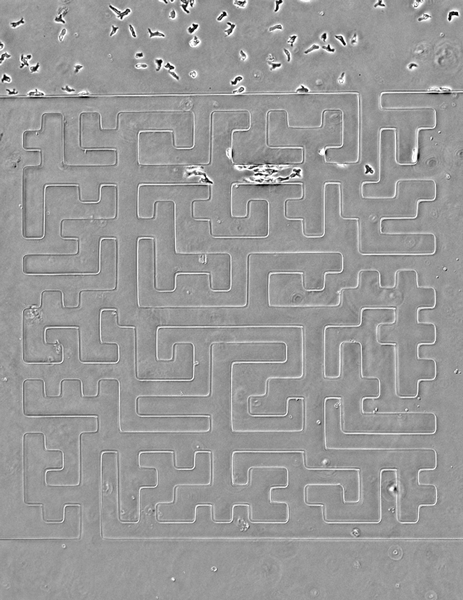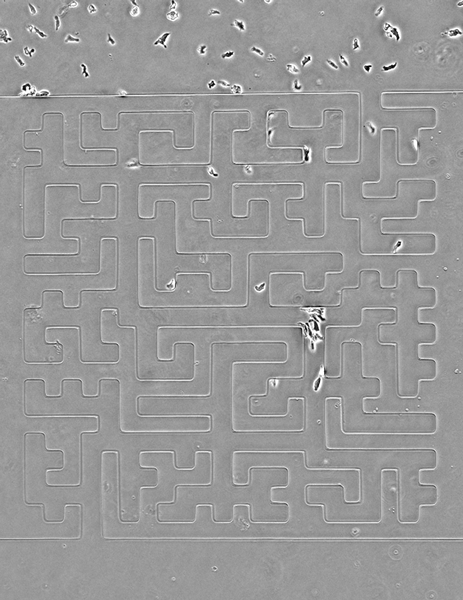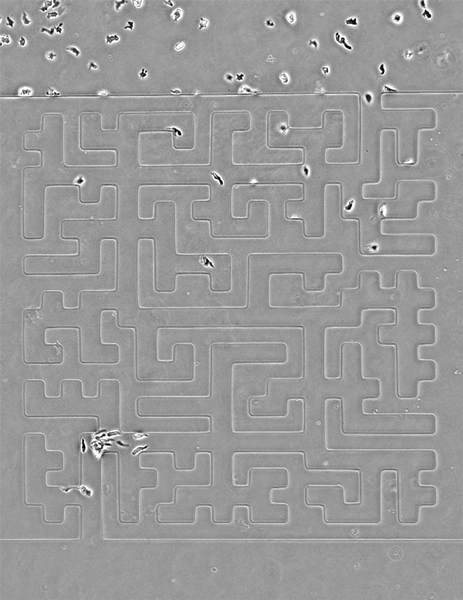
Seen here, the team designed a version of the famous Hampton Court palace maze and built it in microscale (the paths are less than 50 microns wide). Cells are easily able to solve it, better than people, in fact, the researchers note. The team also noticed an imperfection in the microfluidic device that caused a small short circuit; cells easily noticed it and took advantage of a shorter route.
Invest in quality science journalism by making a donation to Science Friday.
Luke Tweedy is a postdoctoral researcher at the Beatson Institute for Cancer Research in Glasgow, Scotland.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. We all know that cells are the basic building blocks of life. Our bodies are made up of trillions and trillions of them. And they all serve a specific purpose. But did you know that cells don’t always stay in the same place. They move around the body. And for a long time now, researchers have been trying to better understand how they move and why. One team of scientists in the UK set up an interesting adventure for two kinds of cells. They put them through tiny mazes to see what would happen.
Joining me today to talk about this research is my guest Dr. Luke Tweedy, a postdoctoral researcher at the Beatson Institute for Cancer Research in Glasgow Scotland. Welcome to Science Friday.
LUKE TWEEDY: Thank you for having me.
IRA FLATOW: You’re welcome. It’s really interesting research. Let’s talk about it. What was the idea here from the beginning?
LUKE TWEEDY: Well, our lab have always been really interested in why cells move and how they move. And we see all sorts of examples in the development of the human body and in disease. For example, when– cancer is particularly dangerous when cells from the original tumor start moving.
And we look at this all the time. And the thing that strikes us is that they always seem to know which way to go. I mean, it’s a very complex environment. And there isn’t necessarily a lot to go on. So where are they getting the information that steers them away from an original tumor or to the right part of the body?
IRA FLATOW: And what other cells besides cancer cells? Are there other cells that move around the body?
LUKE TWEEDY: There are lots, particularly in a developing embryo. So obviously, there are lots and lots of different types of cells that go into make your skin and pigment your skin and develop certain organs. And there are nerves of various types. And huge numbers. I’m sure your listeners are aware of this.
Most of them don’t start in the right place. So when the embryo is developing– when a human embryo is developing or– this is true for any animal, really. There is a lot of migration going on. Cells have to get to all sorts of places. But in a healthy adult human, the obvious example is the immune system. I mean, immune cells patrol your body looking for infection, looking for signs of damage.
And when they find it, they have to signal to all of the other immune cells in your blood, around the peripheral tissues, oh, look, here is something that’s going wrong. And then you find that quite a lot of other cells will come out from rather disparate regions to cause, say, inflammation in that site.
IRA FLATOW: Now, I’m really intrigued at the tact you took to test these cells. You built mazes for the cells. What’s the idea there?
LUKE TWEEDY: Well, we’d been doing a lot of very useful research using just ordinary Petri dishes, right? But we thought to ourselves, a lot of the challenges of navigating in the body are navigating a complex environment. I mean, there isn’t just a nice flat surface to go along. They have to go around all sorts of other developing nerves and blood vessels and tissues.
And so we thought, maybe we could replicate that kind of idea. It’s too complicated to always do this kind of research in, say, an animal. So instead we thought, well, that sounds rather like a maze. Maybe we could literally just make a maze and see how the cells do.
IRA FLATOW: And tell me how big these mazes were.
LUKE TWEEDY: Oh, very small. With some designs, we were fitting maybe 16 or 24 or 36 mazes in a dish that was the size of a fairly ordinary coin. We were using mazes that were about 900 micrometers across. So less than a millimeter.
IRA FLATOW: So these mazes, were they like the kinds a mouse would run? Or are they more like the kinds a human would run in a hedgerow?
LUKE TWEEDY: Well, certainly more like the kinds a human would run. One of the mazes we designed was literally a replica of Hampton Court Palace maze. It’s a famous garden maze in London.
IRA FLATOW: No kidding.
LUKE TWEEDY: Yeah, absolutely.
IRA FLATOW: Whose idea was that?
LUKE TWEEDY: That one was mine, I think. It was a less informative maze than some of the others. We learned an awful lot about how cells make decisions from these mazes. Because of course, mazes are all about making a decision. Do I go left or right here, right? Hampton Court was very showy, and has been a very popular maze for images on web sites. It wasn’t fundamental to our research, I must admit.
IRA FLATOW: Yeah, it’s interesting what you just said. They make decisions. Does that mean they have some kind of intelligence within themselves?
LUKE TWEEDY: No. They are very kind of programmatic in the way they do things. Obviously, there’s a lot of randomness to the environment. But what the cells are doing is trying to read from the chemicals in the environment about which way they might want to go. What’s where? Where might they find more nutrients, say? Or in terms of human biology, obviously, the nutrition is usually supplied by the bloods.
Quite regularly, the dangerous cancers are the ones that start to move toward blood vessels. And to enter the blood and drift to another other part of the body where they can set up disparate tumors. We’re interested in these kinds of mechanisms and these kinds of decisions. But they’re not intelligent. They’re just complex.
IRA FLATOW: Let’s talk more about the starting line of the maze. Do you put one cell at the starting line of the maze? Or do you put a whole group of them together and let’s see how well they work as a group?
LUKE TWEEDY: As it turns out, they kind of need to work as a group. So the system we used was not a path of chemicals through the maze. Instead, we just put them absolutely everywhere. And then, put a group of cells in one starting location. And we saw how they made their decisions as they progressed.
But they absolutely had to work as a group. Our group size varied a bit basically because there’s some randomness in how well they’re able to metabolize– break down these chemicals that are in the environment. But yeah, absolutely. They were working in groups of four and five up to 30 and 40.
IRA FLATOW: And what kinds of cells did you choose? And what reason did you have for choosing whatever cells you chose?
LUKE TWEEDY: Well, we used two different cell types. And the one that we used for the majority of our experiments is soil-dwelling amoeba. They’re called Dictyostelium, or colloquially, Dicty. And the reason we use them is that they’re effectively prodigies at guided migration. They’re very fast. They are very good at reading quite subtle cues. And they’ve been a go to– we call it a model organism for this particular kind of behavior in cells for a very long time.
Also there’s strong evolutionary conservation in the mechanisms that Dictyostelium use with the mechanisms that mammalian cells use. So they are important for understanding broader mechanisms. We also used a pancreatic cancer line, which are obviously much more close to what we are interested in a medical point of view.
But they’re a lot slower to grow. They’re a lot harder to keep. And mazes that the Dictyostelium would solve in an hour and a half, the cancers would take 72 hours to do. So in terms of fitting in enough experiments, we concentrated on Dicty. They’re much kinder to the researchers.
The cancer line did, at least, demonstrate that they behaved in the same way. And so we could, effectively, use the Dicty as a model for these kinds of questions.
IRA FLATOW: So how do you set up the cells for this maze trial? I’m trying to picture this tiny little dish the size of a coin. I would imagine that there isn’t a gun going off and a rush to the finish line like races we might do.
LUKE TWEEDY: No, well, I suspect there’ll be a few cells that don’t wait and cheat a little bit. But what you will end up looking, if you were looking at it yourself, is this dish. And there’s a big rubber block in it. You can’t see the mazes. They’re too small. The details are very tiny. But there are some whopping great holes in that I sat and painstakingly punched with a biopsy punch.
These holes can fit a pipette tip in. And so I’ll just drop a few cells in that way.
IRA FLATOW: Now, did they learn like a mouse does? Did they have trial and error? Do they follow along the walls? How do they find their way around, and what influences them to turn right, to turn left. And if they make a mistake, do they try it again?
LUKE TWEEDY: Well, absolutely, not. Unfortunately, I can’t get them back out to try the same cells in the same maze. I can try the same type of cell maybe the next day on a replica. But they won’t use mazes. I mean, they certainly weren’t learning in that kind of way. What we were interested in is exactly what kinds of decisions they would make. And they reliably– one set of cells one day and another set of cells next day. They would reliably make the same decisions for the same design of maze.
So what we actually did was vary the design of maze in order to answer exactly your second question, what makes them turn left, what makes them turn right? And it turns out that when you fill the environment with these attractive chemicals, they break it down exactly where they are. And a big group of them will break down an awful lot.
And then, you end up with this sort of gradient of low concentration where the cells are and high concentration elsewhere. And then they just follow that gradient of high concentration, chasing wherever they can find more of the chemical. But down very short dead end branches, you could twist them and turn them, but they were very short dead end branches.
By the time they arrived to make the decision, all of the chemical had drifted out and been gobbled up by the cells already. So they never even looked at short dead ends. Whereas, as we made the dead ends longer and longer, it took longer for this attractive chemical to drift out. And so with those ones, yeah, quite a few cells would often go the wrong way.
IRA FLATOW: And how close to what happens in the body when they’re out traveling in the bloodstream or finding other cells? How close is your maze to what’s going on in the body replicating that?
LUKE TWEEDY: It’s a very good question. I would say we are not incredibly close yet. But what we’ve done is develop a framework for understanding these sorts of problems. So now we can introduce additional levels of complexity. I still think we’re not ready to analyze this directly in the body. But we might start introducing specific cell types that are human cell types or mammalian. So we might look at immune cells, we might look at cancers.
And previously, we would have questions about why a cancer metastasized to this or that location. Or why an immune cell chose to cause inflammation in this particular site. There are already some things we know about it, but we can start to dissect it a little more. Or maybe put some into a maze, introduce some of the hormones and the chemicals that we know are involved in these processes.
And we’ll have a better idea of how they cope with it because we are now able to do two things. Both introduce the chemical environment, which we’ve been doing for a very long time, and introduce the topological complexity at the same time. So introduce this complex long-branching structure to them as well.
IRA FLATOW: If I heard you correctly, you said the cancer cells took, what, 72 hours to go through the maze, which is not really sprinting, is it? I mean–
LUKE TWEEDY: No, it’s not.
IRA FLATOW: How does that impact your understanding of them as you are a cancer researcher?
LUKE TWEEDY: I actually started life as a theoretical physicist. Cancer research is a relatively recent change for me. But I’m honestly still quite terrified by them. The degree to which they can be guided in one direction is extraordinary. But I would say two things to that. First of all, the cancer cells made the same decisions as the Dicty, they just took a lot longer over it. So they were actually very good at guidance and understanding guidance, they just weren’t as quick.
And then, the second thing I would say is that three days to travel that kind of distance– I mean, it might seem like it’s quite slow. But that might be the distance to a blood vessel. And then, you have a tumor that lets out these metastasizing cells. And they get to a blood vessel and suddenly you’ve gone from an operable cancer to something that’s going to require radiotherapy and chemotherapy and further analysis.
Honestly, that speed still terrifies me. And I think it’s a very important thing to look at further.
IRA FLATOW: Quick reminder. I’m Ira Flatow and this is Science Friday from WNYC Studios. In case you’re joining us, we’re talking to Dr. Luke Tweedy, a post-doctoral researcher at the Beatson Institute for Cancer Research in Glasgow Scotland who set up a maze for cells to run through.
You mentioned the chemical attractant that you use a sort of the cheese for the rat maze here. Is that also found in the body? And could we use that to fend off the travels of a cancer cell and to be attracted to another place in the body?
LUKE TWEEDY: So it’s an interesting question. There are two different attractants, of course, that we’re using. One for the Dictyostelium. And though that is naturally occurring in the human body, it’s not particularly medically relevant. It has a very different role for us as it does for amoeba. The other one is a signaling lipid called lysophosphatidic acid, or LPA.
And LPA is very commonly found in healthy skin. And we find, actually, that where melanomas develop, you end up with a little bit of a dip in the amount of LPA because they start gobbling it up. We might be able to convince tumors, in the very short term, to stay put little longer by adding LPA. But it has important roles in the body. So I am not sure I would want to dive straight into messing with that.
However, where we know that it’s being degraded, it might be nice to add a little bit more. There is one fundamental problem with that, though. And it’s that LPA also tells cancer cells to grow and divide.
IRA FLATOW: Oh, small detail here.
LUKE TWEEDY: So we’re in a dangerous situation where, on the one hand, yes, you might convince them to stay put a little bit longer. But on the other hand, it’s what’s known as a mitogen. It causes further growth. So we probably don’t want to encourage that aspect of it.
If we could find something that interfered with the receptor without causing it to signal– without causing the cell to understand that it’s getting a growth instruction, then that would be wonderful.
IRA FLATOW: So tell me what your next steps from here are that will help you better understand how these cells move. What do you want to know? What do you need to know?
LUKE TWEEDY: There are a huge variety of ways that we could take this. A lot of what we want to do now is what you’d term translation. So we take a basic biological finding, and then we’ve tried to keep this general. We’ve used amoeba as well as mammalian cells. And then, we want to find some kind of medical application.
My boss at the moment and I are going in slightly different directions with this. I’m interested in the immune and inflammatory implications of it and trying to build devices that analyze the computation of the immune system. So we might better understand what’s going wrong if you get arthritis, for example.
My boss remains a very hardened a cancer researcher and is looking more at that kind of angle.
IRA FLATOW: One last question. You said before that you were terrified by knowing something. What was terrifying you?
LUKE TWEEDY: The rapidity and the certainty with which cancer cells metastasize when you’re watching them under a microscope. We choose metastatic lines. We choose lines that are going to give us an idea of what the worst cancers are doing. And we deliberately select them for worse and worse features in this way. But nonetheless, seeing it happen, I do find it very intimidating.
The very first time my boss saw one of the videos we made he said that he was going to go and make an immediate appointment to have all of his moles mapped. I think understanding diseases like cancer doesn’t make it any more comforting, certainly.
IRA FLATOW: Wow, that’s a great perspective. I want to thank you for taking time to be with us today, Dr. Tweedy.
LUKE TWEEDY: Absolutely. I’m delighted to have come.
IRA FLATOW: Dr. Luke Tweedy, a postdoctoral researcher at the Beatson Institute for Cancer Research in Glasgow Scotland. And if you want to see pictures and videos of the mazes and the cells going through them, you’re in luck because you can head over to our website at sciencefriday.com/cellmaze.
Copyright © 2020 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Kathleen Davis is a producer and fill-in host at Science Friday, which means she spends her weeks researching, writing, editing, and sometimes talking into a microphone. She’s always eager to talk about freshwater lakes and Coney Island diners.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.