Personalized Immunotherapy Shows Promise Beyond Cancer
17:23 minutes
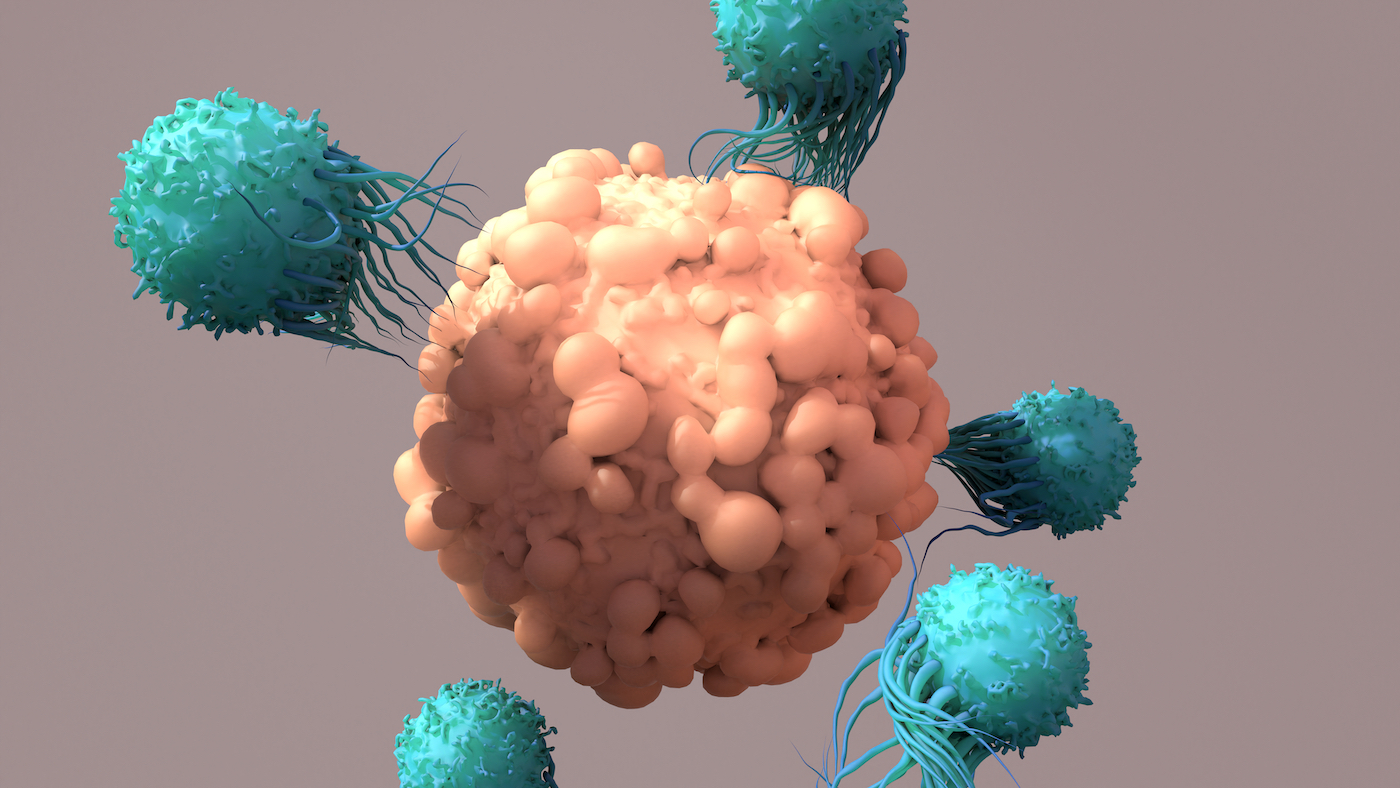
CAR T cell therapy, a type of immunotherapy in which a patient’s own immune cells are modified to create a hybrid immune cell that destroys cancer cells, was first developed over a decade ago.
Now, researchers are continuing to find success in treating new types of blood cancers with the therapy, and are working on applying the technology to solid state cancers like those of the pancreas and brain.
Scientists are also at the early stages of testing CAR T cells to treat autoimmune diseases like multiple sclerosis (MS) and lupus.
Ira talks with Dr. Carl June, one of the pioneers of CAR T cell therapy, a professor of immunotherapy and director of the Center for Cellular Immunotherapies at the University of Pennsylvania, based in Philadelphia.
Invest in quality science journalism by making a donation to Science Friday.
Carl June is a professor, Immunotherapy and the director of the Center for Cellular Immunotherapies, at the University of Pennsylvania in Philadelphia.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. Last week, we were talking about cancer vaccine research. We’re continuing our look into the latest in cancer therapy, and this week, CAR-T cell therapy, in which a patient’s own immune cells are modified to create a hybrid immune cell that destroys cancer cells.
CAR-T cell therapy is not new. It’s been around for over a decade. But researchers are continuing to find success in treating new types of cancer with the therapy. And they’re working on many more.
And on top of that, scientists are starting to investigate the utility of this therapy for conditions other than cancer– autoimmune diseases, like multiple sclerosis and lupus. Joining me now is Dr. Carl June, one of the pioneers of CAR-T cell therapy. He’s professor of Immunotherapy and director of the Center for Cellular Immunotherapies at the University of Pennsylvania based in Philadelphia. Welcome back to Science Friday.
CARL JUNE: Thanks, Ira. It’s great to be back.
IRA FLATOW: Nice to talk to you. OK, let’s start with a quick refresher course. Tell us about what CAR-T cells are and how the therapy works.
CARL JUNE: Well, sure. So a CAR-T cell is a chimera. And all those Greek mythologies out there, a chimera was a fusion of three animals– a lion, a goat, and a serpent. And a chimeric T cell is a fusion of a B and a T cell.
Over the years, the public didn’t know what a B and a T cell was. We first learned about T cells when HIV came out. And HIV kills T cells, and then people lose their immune systems. And then B cells, I think, we’ve all learned about what they do over COVID. B cells make antibodies.
And we’ve all learned about how, for instance, your antibody can spike, protein for instance, can protect you against COVID. So B cells make those antibodies. And T cells don’t. A CAR-T cell is a chimera, a fusion of a B and a T cell, so that now you can have a T cell that can do what T cells do, but they also can have an antibody in there, as normally what would be done by a B cell.
IRA FLATOW: So these are sort of designer cells that each individual patient has. You have to create that from the patient’s cells, correct?
CARL JUNE: Yeah, that’s one of the parts that’s a big paradigm shift of this. The pharmaceutical industry heretofore has always made drugs you know where one shoe size fit all, which has a lot of economy of scale. You can make one drug that’s for everyone.
In this case, the CAR-T cells are made individually or bespoke for each patient. So the patient actually donates blood. The cells are shipped to the manufacturing center, and then shipped back after the manufacturing as CAR-T cells, and then given as a simple blood infusion.
IRA FLATOW: And the last time we had on the show, we discussed the exciting milestone that two patients you treated with CAR-T cells a decade ago are still in remission, a cure even. And now there’s a new generation of CAR-T cell therapies that are more potent and attack different cancers beyond the blood cancers? Is that correct?
CARL JUNE: Yeah, that’s correct. It’s rapidly evolving from what was, basically, an academic experiment in a few laboratories to now it’s an industry that’s world wide. Initially, the CAR-T cells we made were approved for leukemia, acute leukemia, which is not a common disease. And it’s a blood cancer. And as you mentioned, those initial patients appear to be cured.
Now, over this last year, CAR-T cells have been approved also for myeloma, which is the most common blood cancer in adults. The major significance of this is it shows that it’s not a one-trick pony. So this shows that it’s a generalizable strategy, that you can change the warhead that comes out of the B cell at will, and then target virtually any cell in your body.
IRA FLATOW: Even cells in your brain like, glioblastoma?
CARL JUNE: Yeah, that’s a very exciting area. And in fact, Marcella Maus, who’s at Harvard now, has had trials targeting glioblastoma. And there is a recent trial from Stanford targeting another molecule called GD2 in a childhood brain cancer. So there’s even into the brain, as you mentioned.
IRA FLATOW: Hmm. What about one really deadly cancer, pancreatic cancer? Any hope there?
CARL JUNE: Well, pancreatic cancer has been, I think, safe to say, since my days in medical school, the worst of the worst. It has not responded to so-called checkpoint therapies that have previously revolutionized cancer therapy and now are first line for lung cancer and melanoma and other cancers. They just don’t work in pancreatic cancer. And now there are CAR-T cells that work in mice in laboratory models for pancreatic cancer. But as of yet, the responses are still disappointing in humans. And there’s a large need for research there.
IRA FLATOW: And I understand that CAR-T cells are now being tested in early clinical trials to treat autoimmune disorders too.
CARL JUNE: Yeah, that’s a really exciting area. Autoimmune disease was found to be an overreaction of the immune system against your own normal body tissues. And that’s, in fact, at some level, what we try to provoke with cancer immunotherapy, to destroy a body tissue, in this case, a cancer tissue. Autoimmunity is up between 10% and 20% of adults in the US. It’s been treated but never cured before.
The great news has been that biologics have come out to treat diseases such as arthritis and multiple sclerosis. But they haven’t been curative. That adds up to a significant expense. And frankly, just the patients would prefer cure therapies. So there are exciting trials open now in lupus, which is one of the systemic autoimmune diseases.
And a very intriguing case report published in the New England Journal of Medicine about a year ago showed a 20-year-old Asian woman who was treated with a single infusion of CAR-T cells, and her disease went into remission. And I’ve spoken to the investigators in this– it’s a first in human phase I trial. That complete remission is ongoing. And they now have multiple other patients like that.
IRA FLATOW: Wow.
CARL JUNE: So this is really an important early stage area of research.
IRA FLATOW: Is that because– as you said before, the success is because these are designed specifically for a person’s genetic makeup?
CARL JUNE: Yeah, so they come from our own T cells, which each person has. And unless you have an identical twin, the only person who can ever be a donor for you at this point is yourself. So it’s not like red blood cells, where we have– if you’re O-negative, you can be a universal donor. So T cells, at this point, all have to come from the patient themselves.
And then they can now be modified at will with gene engineering, either genetic editing using technologies such as CRISPR and Cas9, or with insertion techniques to knock in genes that make the T cells that you have to be weapons that can target virtually any cell in the body at will. It’s really a remarkable new advance that came from the confluence of many new genetic technologies.
IRA FLATOW: Does this mean that we can develop new drugs a lot faster than the over a decade-long time periods we have now?
CARL JUNE: Yeah, that’s been something we’ve learned. So now that we have, for instance, a lens looking back 10 years from initial patients we treated with leukemia– so they were given a single infusion of their own T cells that were CAR modified cells. They’ve lasted 10 years in those patients. And they have not caused any adverse responses, meaning they’ve been safe.
And now thousands of patients have been treated with CAR-T cells, where they’re manufactured from their own cells. So as a group, cell therapies, if they come from your own cells, appear to be very safe. We’ve known for many years that cancer drugs that are cytotoxic and break DNA as a mechanism of action, they actually can cause cancer. And so far, that has not occurred with the patient’s own T cells.
So when you go back to the drawing board to make a new CAR-T cell, as was done for myeloma that I mentioned that was just approved in this last year, that took less than five years to go from the drawing board to an FDA-approved product. And the usual pharmaceutical cycle, if you look it up, would say it’s 10 to 15 years to make a new drug. So now that we have technologies that are validated with manufacturing cells, I think the drug cycle time for the first time is going to be shorter than the actual patent duration.
IRA FLATOW: That’s bad news for drug companies.
CARL JUNE: It is. But it’s great news for the patients. It means that it encourages innovation. And in the past, many drug cycles– the pharmaceutical industry could rely on patent protection before they needed to have a new drug come out. And now what happens is it’s more like cell phones. Each year, if your iPhone is better, you’ll switch to that one. And it doesn’t matter how much Apple has on patent protection because what drives innovation in the battle between Samsung and iPhones is innovation.
IRA FLATOW: So let’s talk about how expensive all of this still is. How much does a typical CAR-T cell therapy cost?
CARL JUNE: So that’s, right now, the Achilles heel of this area. Because they’re made one by one for each patient, they’re much more expensive than previous drugs, which are made in batches for all patients at one time. And so the initial CAR-T cell trial prices for leukemia were around $400,000 per patient.
The one bright side on that is that it actually came with a guarantee that it would work. So normally, when you get treated, say, with cancer therapies, there’s no guarantee that it works. And the hospital and the patient’s insurance companies pay for this regardless. Now, CAR-T cells are given– there is, in leukemia, a guarantee that it will work. And if you’re not put in remission, then the price is rebated.
IRA FLATOW: I never heard of anything like that before.
CARL JUNE: Yeah, and that’s because the initial remission rates were– in refractory patients where they literally had weeks to months to live, there was an 80% and 90% complete response rate, which hadn’t really ever happened in refractory cancers like that. So when you have expensive therapies, they need to have very potent effects to make them worthwhile. And so there’s a lot of research now to make them cheaper so that they’re not so expensive.
But the sad fact is now that even patients with myeloma, which used to– when I was in medical school, the survival was two or three years. Now it’s 8 to 10 years. But the textbooks all still say it’s incurable. And over that time, patients spend over $1,000,000, or their insurance companies do, before they, unfortunately, have demise from the cancer after many different kinds of therapies are given. And so if you have an expensive therapy that’s 400,000 now, it still can be economically cheaper than what we do right now, which is death by 1,000 cuts from many different therapies given month by month over the years.
IRA FLATOW: Interesting, interesting. I imagine since this is basically handmade for each patient, there’s got to be a long line of patients who have heard about this and are waiting on line to get their CAR-T cell therapy.
CARL JUNE: So unfortunately that’s true. And I think this is– many new technologies often are limited in production. So at this point, the manufacturers of CAR-T cells on this one-by-one manufacturing cannot meet the demand. And there is a waiting list.
CAR-T cells are in some ways similar to the, I think, automobile manufacturing, where those cars initially made by Henry Ford were put together initially by hand, one by one on assembly lines. And then now, most automobiles are mostly assembled by robotics. The most expensive part of manufacturing CAR-T cells is human labor with highly trained technicians and scientists. And this needs to become automated, just as automobiles have.
IRA FLATOW: Does this manufacture, the individual CAR-T cells, do we send them overseas to laboratories? Or do we do them in the States?
CARL JUNE: So at this point, they’re done in the States. And then there are manufacturing centers in Europe. There may well be economic competition. Right now as you know, we outsource a lot of our IT needs overseas to Asia. And labor may be cheaper, and similar in South and Central America.
IRA FLATOW: This is Science Friday from WNYC Studios. Listeners to this show know that we here at Science Friday can’t get enough information about the microbiome. And I understand that some recent research from your colleagues at UPenn and Memorial Sloan Kettering shows that the microbiome may actually play a role in how well CAR-T cell therapy works.
CARL JUNE: It’s a remarkable response– I mean, end result. So Melody Smith who is at Memorial Sloan Kettering, and Marco Ruella, a colleague of mine here at the University of Pennsylvania, looked at our patients between New York City and Philadelphia and found that their microbiome had a major impact on how they responded to CAR-T cell therapy when they had various blood cancers. And so this was astonishing to me because this is something– we grow these cells in the lab, and you say, how can the microbiome in your GI tract affect these cells that are given to patients? And we’ve now found in mouse models that, in fact, it validates what we’ve found in the human patients treated in New York City and Philadelphia.
IRA FLATOW: So if you have a better microbiome, in other words, a better kind of bacteria growing in your gut, you might do better with CAR-T cell therapy?
CARL JUNE: That’s exactly what this research suggests, and published in Nature Medicine. And I think there will be now attempts to modify– there are various probiotics and other things to do this at will that may well improve the response rate in patients.
IRA FLATOW: I’m reminded– because of a I’m of a certain age, I remember when antibiotics after World War II were called miracle drugs. Do you feel that excited about CAR-T cell therapies, the potential for them, that we are in a paradigm shift on that scale?
CARL JUNE: I think without a doubt. My colleagues here earlier this year published, I think, quite remarkable responses in repairing heart damage with CAR-T cells. And that– so there’s now been several studies on that. Normally, if you have a myocardial infarction, it leaves a scar in your heart. And if it’s a big enough scar, your heart really doesn’t work as a pump anymore.
And now it’s been found that you can make the regeneration of the heart muscle much better. This is in mice. That’s just one example. I think we’re going to see cell therapies that fix scars in the lung, and hopefully, as we’ve talked, autoimmune diseases like multiple sclerosis and lupus and arthritis.
IRA FLATOW: Well, I can only wait and hope and look around for that day to come, Dr. June.
CARL JUNE: Well, I think it’s a very exciting time. And now it’s global. It’s gone from just an academic curiosity to– it’s really been exciting to see this in evolution.
IRA FLATOW: Dr. June, thank you for taking time to talk with us about this exciting research.
CARL JUNE: It’s all my pleasure. Thanks, Ira.
IRA FLATOW: Dr. Carl June, Professor of Immunotherapy, Director of the Center for Cellular immunotherapies at the University of Pennsylvania in Philadelphia.
Copyright © 2022 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Shoshannah Buxbaum is a producer for Science Friday. She’s particularly drawn to stories about health, psychology, and the environment. She’s a proud New Jersey native and will happily share her opinions on why the state is deserving of a little more love.
Ira Flatow is the founder and host of Science Friday. His green thumb has revived many an office plant at death’s door.