A See-Through Squid Success Story
12:15 minutes
Adult octopuses have about 500 million neurons, which is about as many neurons as a dog. Typically, more neurons means a more intelligent and complex creature. But it’s a bit more complicated than that. Unlike dogs, or even humans, octopuses’ neurons aren’t concentrated in their brains—they’re spread out through their bodies and into their arms and suckers, more like a “distributed” mind. (Scientists still haven’t quite figured out exactly why this is.)
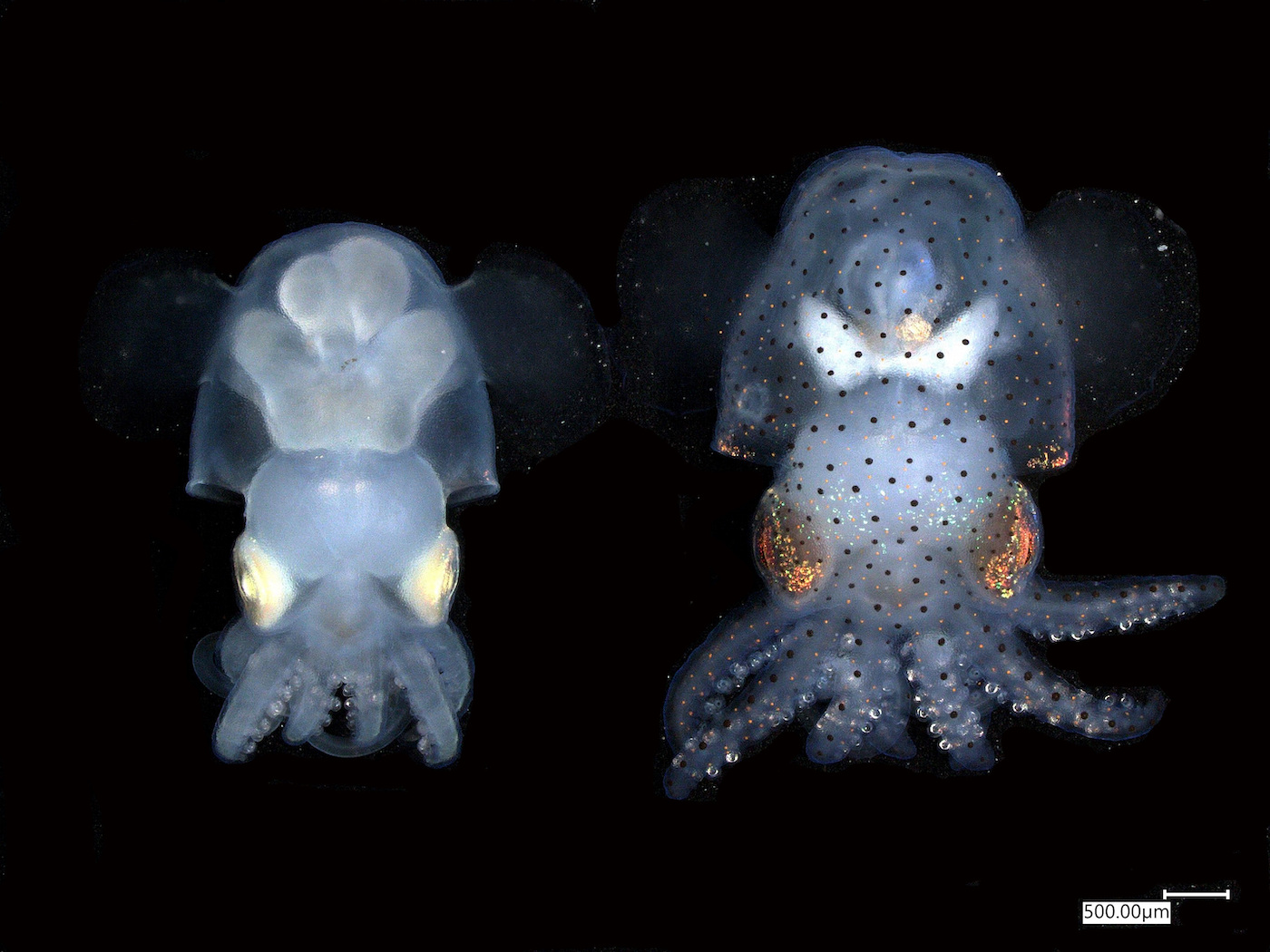
And that’s just the tip of the iceberg, in terms of unanswered cephalopod questions. Now, researchers have successfully bred a line of albino squid that were first engineered using CRISPR-Cas9 gene editing technology, creating a see-through squid.
Their unique transparency allows scientists to more easily study their neural structure, and a whole lot more.
SciFri experiences manager Diana Plasker talks with Joshua Rosenthal, senior scientist at the Marine Biological Laboratory, in Woods Hole, Massachusetts, an affiliate of University of Chicago, about this see-through squid success story.

Josh Rosenthal is a senior scientist at the Marine Biological Laboratory, University of Chicago, in Woods Hole, Massachusetts.
IRA FLATOW: This is Science Friday. I’m Ira Flatow. It’s that most wonderful time of the year. Of course, I’m talking about Cephalopod Week. We’re cephalo-brating our favorite invertebrates. I’m talking squid, octopus, cuttlefish. And joining me now to share some of the latest ceph science is SciFri Experiences Manager Diana Plasker. Yo, Diana.
DIANA PLASKER: Howdy, Ira. So did you know that adult octopuses have about 500 million neurons, which is about as many neurons as a dog has?
IRA FLATOW: But how well can they catch a Frisbee?
DIANA PLASKER: I’m not sure anyone has ever tested it. But who knows? The future is vast. So typically, more neurons means a more intelligent and complex creature. But it’s complicated. Unlike dogs or even humans, though, octopus neurons aren’t concentrated in their brains. They’re spread out through their bodies, into their arms and suckers, more like a distributed mind, you might think.
And scientists still haven’t quite figured out why exactly this. It’s just the tip of the iceberg in terms of unanswered cephalopod questions, which is why I’m really excited about a new study that came out this week. Researchers have successfully bred a line of albino squid that was first engineered using CRISPR Cas9 gene editing technology, so basically a see-through squid.
IRA FLATOW: No. A see-through squid?
DIANA PLASKER: That’s right.
IRA FLATOW: Wow.
DIANA PLASKER: Yeah, and this way we can more easily study their neural structure and a whole lot more. Here to tell us more about this see-through squid success story is my guest, Joshua Rosenthal, Senior Scientist at the University of Chicago’s Marine Biological Laboratory based in Woods Hole, Massachusetts. Josh, thanks for joining us today.
JOSHUA ROSENTHAL: Well, thanks very much for having me.
DIANA PLASKER: So to start off, what do see-through squid have to do with getting a more detailed understanding of cephalopod brains?
JOSHUA ROSENTHAL: Well, one of the basic approaches that neuroscientists use to study brain activity is to actually look at it. And you can do that by what we call putting a fluorescent molecule in the brains of animals that will become fluorescent only when the nerve cells are active. And in order to look at that fluorescence, you have to be able to see it. It can’t be obstructed by things like pigmentation.
And so by generating basically a see-through squid, we’ve developed a line of squid now where you can actually see the neurons in action in large numbers of them at once. And then we can start asking basic things on how this really incredible nervous system works.
DIANA PLASKER: That’s amazing. So let’s talk a little bit about how you actually did this. So you use CRISPR gene editing technology to knock out the squid’s pigmentation. Tell me a bit about the genes you identified and what the process was to make that happen.
JOSHUA ROSENTHAL: Yeah, that’s a great question. So this is basically a follow up to a study we did a couple of years ago, where we knock out pigmentation in our local squid here. And the issue with our local squid here is it’s a really interesting animal, but you can’t culture it. We just can’t keep it alive in the lab.
DIANA PLASKER: And by culture you mean actually breed in the lab?
JOSHUA ROSENTHAL: Exactly. And what we were trying to do now was basically not only knock out pigmentation in that first generation, but also be able to breed successive lines because that ultimately is going to be much more useful. So let’s say we said, let’s make it easy. Let’s knock out the exact same gene we did from our local squid into this new one, called euprymna berryi. The gene is called TDO, or tryptophan 2, 3 deoxygenase, in complex terms.
DIANA PLASKER: An easy name.
JOSHUA ROSENTHAL: Yes. And we did it now in our new model, euprymna berryi. And nothing happened.
DIANA PLASKER: That sounds like science, yeah.
JOSHUA ROSENTHAL: Yeah, and so we said, what could have gone wrong? And so finally, after scratching our head, we looked really more carefully into this pathway of similar genes and found that in vertebrates, in vertebrates, like us, there are two genes that produce proteins that can do the same reaction. One is TDO, the one we targeted. And the other is called IDO.
So basically, my collaborator on this whole study, Carrie Alberton, went and she started digging deeply into other invertebrate genomes to see if IDO could possibly be in some cephalopods. And lo and behold, she found that they had this other gene that, in us, at least, catalyzes the same reaction. So we then made two different CRISPR guides targeting two different genes, co-injected them at the same time, and lo and behold, we were able to knock out pigmentation.
DIANA PLASKER: Amazing. So it was a many-stepped process. Can you tell me a bit more about why you chose this specific species of squid? So the other local squid wasn’t working out. But the common name for this one is the hummingbird bobtail squid. Why that squid?
JOSHUA ROSENTHAL: Cephlopods are notoriously very difficult to culture. When you’re looking for a new model, there are certain characteristics that are advantageous. One of them is that you can culture them through their lifecycle, obviously. The other is that that lifecycle is pretty short, in that they reach sexual maturity quickly. And that’s just pragmatic.
So this hummingbird bobtail squid, it reaches sexual maturity in about three months after hatching. So that’s pretty good as a cephalopod. The genome has recently been sequenced. That’s an advantage too. And basically, a generally docile demeanor is good. And finally, especially if you can put more than one animal in a tank, it saves space. Certain cephalopods, like octopus, are notoriously cannibalistic. And that’s not so good. And then finally, the last thing is they should be small. These are an inch, inch and a half long.
DIANA PLASKER: Amazing. You’re talking about a small inch-long squid. It buries itself in the sand. Can you describe for our listeners what these squid look like before the gene editing happens?
JOSHUA ROSENTHAL: Yeah, they basically are– as you said, they’re small. They bury in the sand and stay hidden for most of the day. And then they come out and hunt little shrimp in our systems at night. They’re fairly darkly pigmented. These are pigmented sort of a dark reddish dark brown on the outside. Some people, people who are used to looking at things like octopuses or cuttlefish that do this tremendous camouflage think they’re, frankly, a little boring. But they have these characteristics that make them great for culture in the lab.
DIANA PLASKER: Very cool. And so what do they look like when they’re albino? You can see through them. So how does that change the way that you actually see them with your eyes and maybe with a microscope?
JOSHUA ROSENTHAL: Yeah, so they’re exceptionally transparent when you knock out this pigment. For instance, with my naked eye, I can look through and see the main parts of the nervous system, which is tremendous. However, there is one caveat here, is when you knock out this pigmentation gene, it’s lethal eventually. And we think it’s because they can’t hunt effectively.
DIANA PLASKER: Well, so one of the fascinating things about cephalopods that people probably really like and are curious about is that they can camouflage their surroundings. They can change shape and colors. If you knock out the genes for pigmentation, can you still observe that process?
JOSHUA ROSENTHAL: No. But I think it might be useful for understanding that process better. So for instance, the camouflage itself is controlled by these organs in their skin called chromatophores. And those chromatophores are controlled by nerves, by nervous activity. Actually, the chromatophores are expanded and contracted with specialized muscle, which are then innervated by nerves that come directly from their brain. And so by knocking out the pigmentation in these chromatophores, I think we’ll be able to better see the underlying neural activity because it would have been blocked.
DIANA PLASKER: So as you mentioned, these lab raised squid, they don’t actually reach adulthood. What are some of the benefits and drawbacks of studying these young squid?
JOSHUA ROSENTHAL: One of the benefits is essentially because they’re small– these things are several millimeters long– they fit much better under the common microscopes people use for imaging neural activity. It sounds small, but an inch, inch and a half adult would not fit very conveniently in a chamber under a microscope. So it’s almost beneficial to have these little animals to be able to image.
Some of the drawbacks is they’re not exactly the same as an adult. So their behavior is still– they’re nervous systems are still developing to some extent. So we are limited a little bit and what kind of questions we can ask.
DIANA PLASKER: We’ve talked a lot specifically about these animals. But I want to zoom out a little bit. What are some of the big questions this line of genetically modified squid might help you and other scientists better understand about cephalopods?
JOSHUA ROSENTHAL: One of the very intriguing things is from the aspect of neuroscience and how you make a behaviorally complex sophisticated organism. Almost all of the complex organisms that we know evolved along a common evolutionary branch. That’s us vertebrates, complex birds, mammals.
So essentially, if you look at, let’s say, what makes a bird smart or what makes a muskrat smart or what makes a rat smart, you’re going to be looking at a lot of common derivatives of the same innovations. Cephalopods did this along their own trajectory. They’re more similar to clams and oysters than they are to anything along the vertebrate tract.
So they enable us to ask a really unique question. And that is what elements do you need to create a sophisticated nervous system that are in common between these two branches? And what are some of the individual innovations between these two groups? I think the stuff I work on in RNA editing is another example, where cephalopods create unbelievable diversity in the molecules that drive their nervous behavior, much more than we have through RNA editing. And so how do they use that to good purpose?
DIANA PLASKER: And what would it mean to science as a field to accept cephalopods, like these little hummingbird bobtail squid, as model organisms, like mice or fruit flies?
JOSHUA ROSENTHAL: We still have a long, long, long way to go to be able to look at things with the sophistication you can with, let’s say, a fruit fly or a zebrafish or a mouse. People have focused on these few organisms. And we know a lot about a few organisms. And there’s all this biological diversity out there of these new different organisms doing incredible things that we understand very little about. And so in order to do that, we have to extend these models out beyond the few that people commonly use.
DIANA PLASKER: Yeah, well, I feel like we’ve gone a long way just today in this conversation, Josh. Thank you so much. That’s actually all the time we have. And I’d like to thank you for being here with us today. Joshua Rosenthal, Senior Scientist at the University of Chicago’s Marine Biological Laboratory based in Woods Hole, Massachusetts. Thanks so much for being here, Josh.
JOSHUA ROSENTHAL: Thank you very much for having me. It’s a pleasure to be on Cephalopod Week.
DIANA PLASKER: For Science Friday, I’m Diana Plasker.
IRA FLATOW: Thanks, Diana. That was really fascinating. And if you still can’t get enough cephalopod facts, we’ve got some really cool Cephalopod Week events planned. Diana, tell us what’s in store.
DIANA PLASKER: Don’t you mean what’s in shore? I’ll jet along to the point, Ira. The Cephalopod Week cephalo-brations are just getting started. We are hosting a whole series of events over the next few days. So if you’re in Miami, Houston, Atlanta, or Thousand Oaks, California, grab those tickets before someone else with eight arms gets there first. You can find out more on our website, sciencefriday.com/octopus.
IRA FLATOW: That’s right. That’s sciencefriday.com/octopus.
Copyright © 2023 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
Diana Plasker is the Senior Manager of Experiences at Science Friday, where she creates live events, programs and partnerships to delight and engage audiences in the world of science.
Shoshannah Buxbaum is a producer for Science Friday. She’s particularly drawn to stories about health, psychology, and the environment. She’s a proud New Jersey native and will happily share her opinions on why the state is deserving of a little more love.