Grade Level
3 - 8
minutes
15 min - 1 hr
subject
Chemistry
stem practices
Developing and Using Models
Activity Type:
Household materials, atoms, build a model, molecules, After School Activity
Take a look at these two pictures of mountains. What do you notice? How are the mountains the same? How are they different?

You may have noticed that the rock formations are very different colors. The rock formations on the left are from Red Rock State Park in Texas, and on the right is El Capitan Mountain in Yosemite National Park in California. The colors are so different thanks to one crucial element: oxygen.
What Are Elements And Compounds?
Elements are the chemical building blocks of matter. Everything in the universe that takes up space is made of elements. There are 92 elements found in nature and another 20 or so that scientists created in a lab.
When elements combine, they form compounds. For example, water is made of two atoms of the element hydrogen, and one atom of the element oxygen. That’s why it’s often denoted as H2O. A chemical reaction is a process in which two or more substances—elements or compounds—are converted into a new substance. When atoms combine, we call that a molecule.
What does this have to do with the mountains in the picture? The rock on the left contains a lot of iron. The iron reacts with oxygen in the air, turning the rocks red. This reaction is called oxidation, the same process that rusts metal.

Build Your Own Compounds
A great way to understand chemistry is to use models representing atoms and molecules to see how they fit together. You will use plastic construction or building bricks, like LEGO bricks, as atoms of different elements. These models are based on the work of MIT Edgerton Center and LEGO. If you enjoy this activity, there are many more lessons you can try.
Materials:
- Several 1×2 building bricks in white for hydrogen. (It’s the smallest atom.)
- Several 2×4 building bricks in four different colors
Suggested colors:
– black: carbon
– red: oxygen
– blue: nitrogen
– orange: phosphorous
Safety note: Bricks present a choking hazard for young children. Please keep bricks out of reach of children under three years of age.
If you don’t have bricks of the same size or color mentioned above, substitute with what you have. If you don’t have any building bricks at all, try using clay or gumdrops with toothpicks instead.
For this activity, you’re going to focus on just five elements that were incredibly important to shaping the environment on our planet: carbon, hydrogen, oxygen, nitrogen, and phosphorus. They occur in nearly every organism on Earth.
Modeling The Components In Air
An atom is the smallest possible piece of an element. When atoms bond, they make molecules. Many molecules form new compounds when different elements bond together. However, some atoms combine into elemental molecules where two of the same elements combine. Hydrogen, oxygen, and nitrogen form elemental molecules.
Let’s start by making some elemental molecules:
- Select two blue bricks.
- Attach the bricks, offsetting them so that the top brick overhangs the bottom brick.
- Make seven more nitrogen molecules.
- Select two red bricks and put them together in the same way you did with the blue bricks.
- Repeat to make a second oxygen molecule.
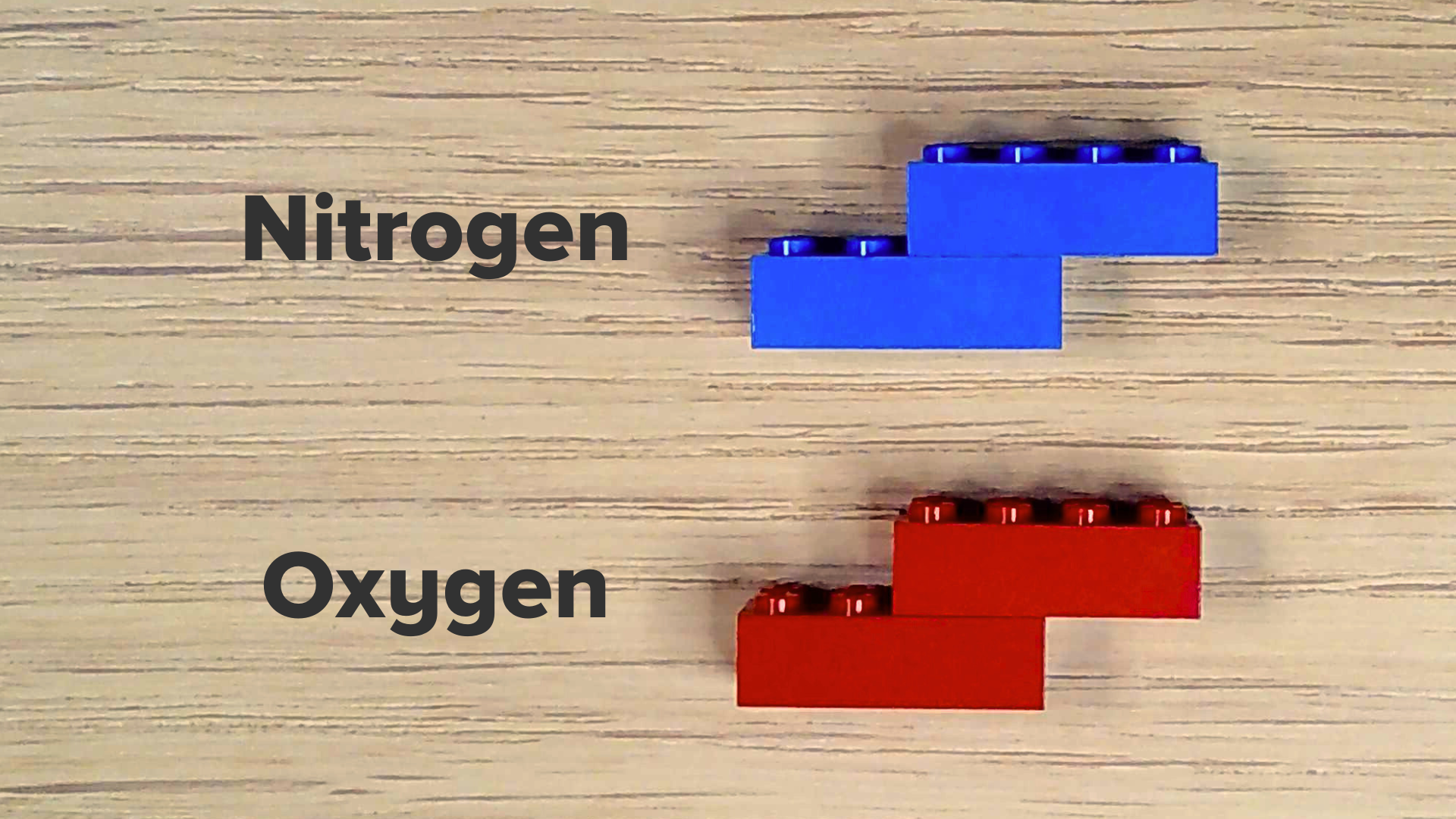
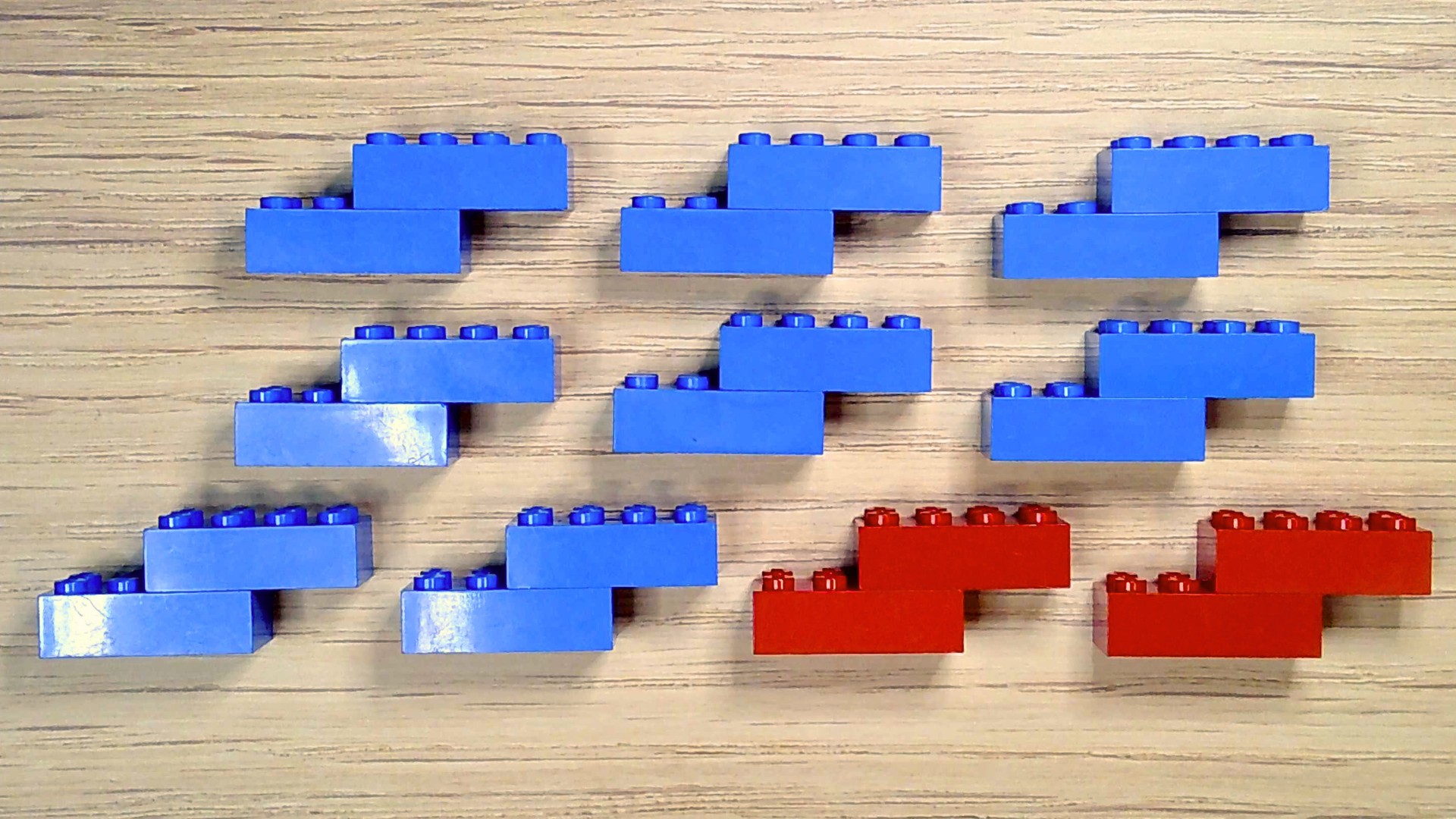
You should now have ten molecules: eight blue nitrogen and two red oxygen. Congrats! You’ve made air, which is almost 80% nitrogen and 20% oxygen.
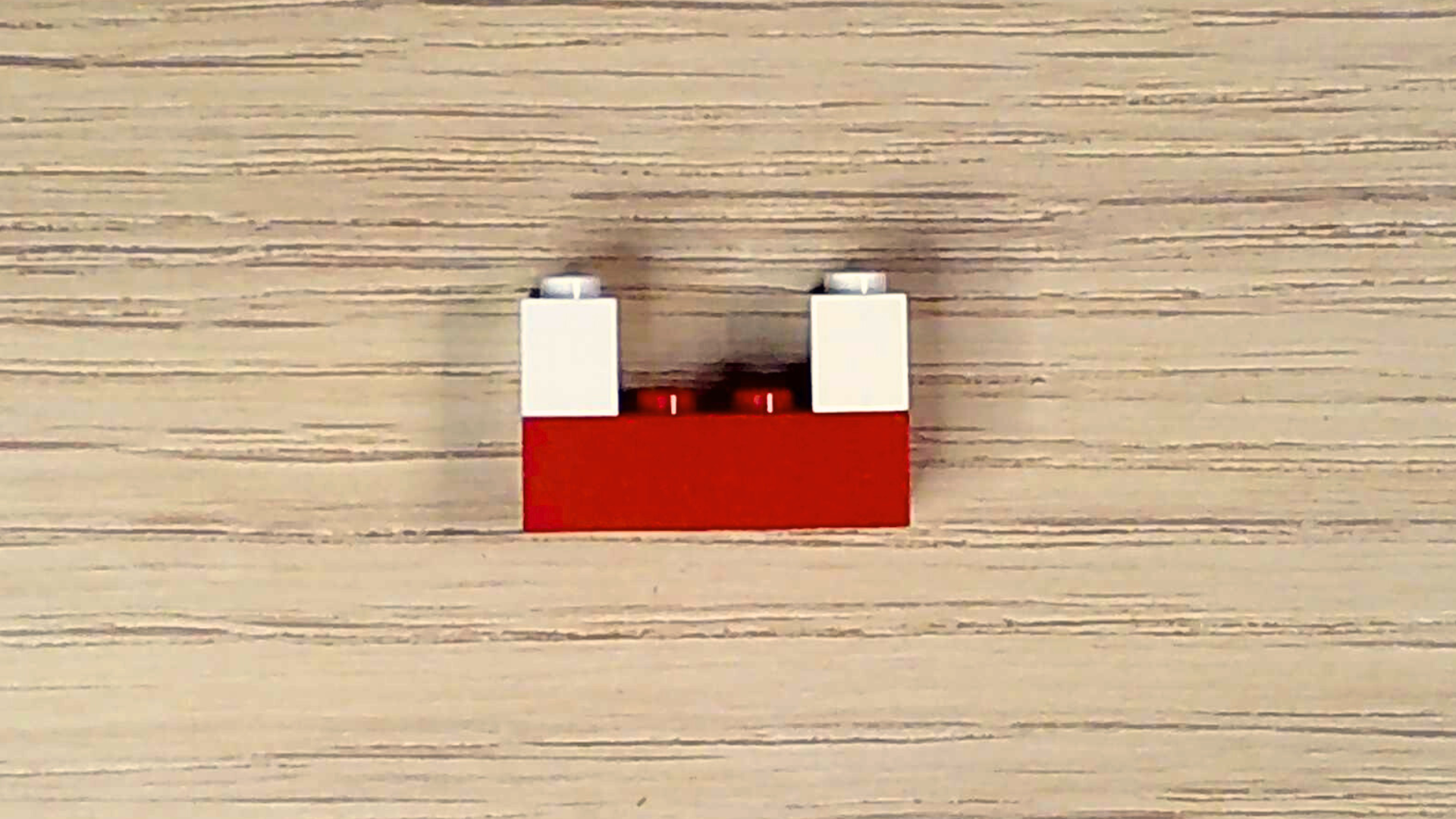
Since air also contains a tiny bit of water vapor, make a water molecule too.
- Select one red brick and two white bricks.
- Holding the red brick lengthwise with the studs facing upward, attach a white brick to either end of the red brick.
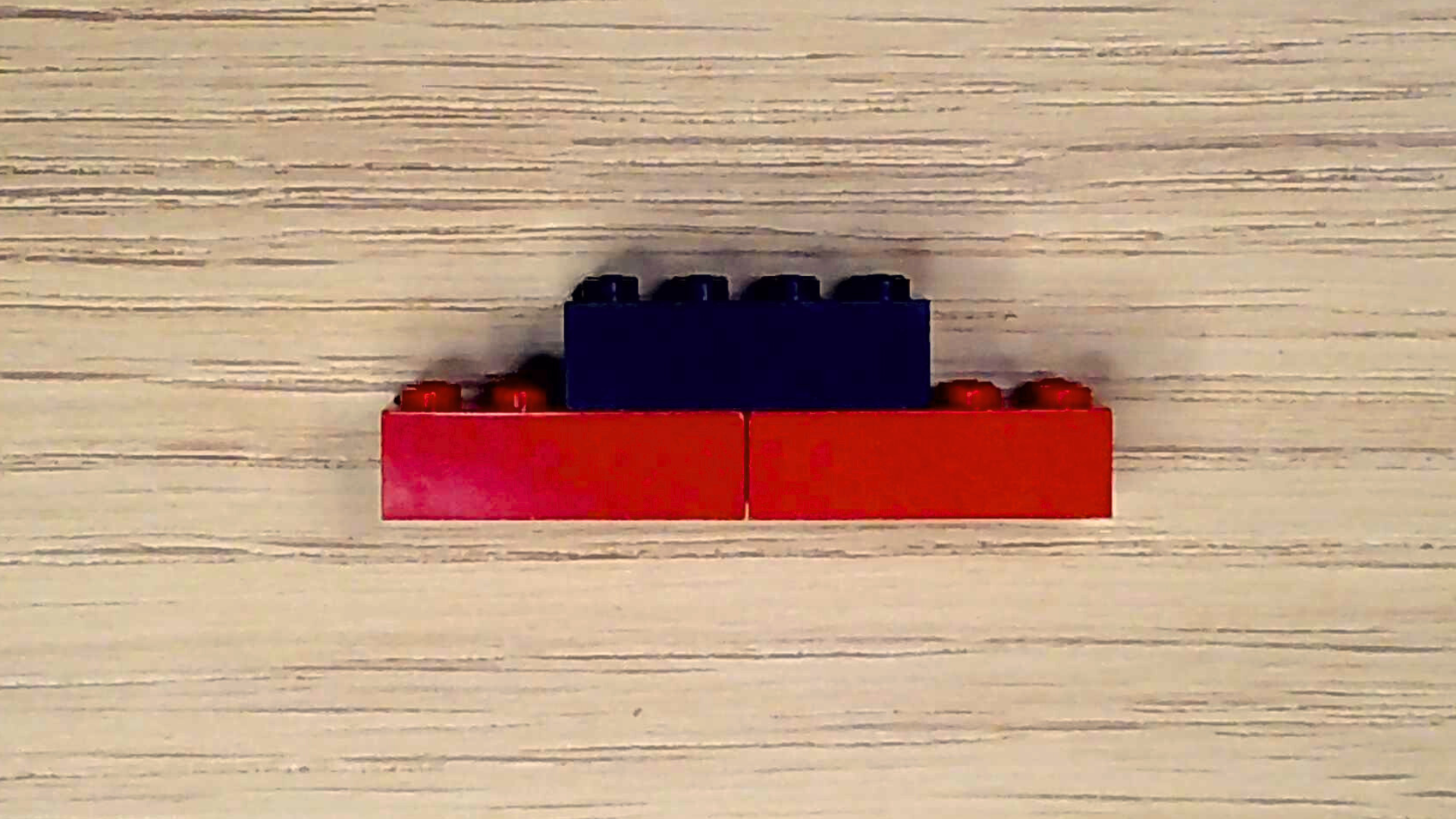
Great! Your air needs just one more thing: carbon dioxide. Carbon dioxide is a waste product that living organisms—like you—produce when they make energy. Plants take in carbon dioxide and, through photosynthesis, make oxygen. Let’s make a carbon dioxide molecule.
- Select one black brick and two red bricks.
- Align the two red bricks next to each other lengthwise with the studs facing upward.
- Center the black brick over the place where the two red bricks meet and attach them.
Together, these molecules make the air we breathe.
Creating A Molecule Group With Phosphorus
Phosphorus is an element found in rocks and soil, but not air. It’s in molecules like DNA and RNA, your genetic blueprints, and ATP, the chemical energy you use to fuel your body. Alone, phosphorus is very reactive, even explosive. So, it bonds to other elements to form a more stable phosphate group. Let’s build it!
- Pick out four red bricks and one orange brick.
- Align the two red bricks next to each other lengthwise, with the studs facing upward.
- Center the orange brick over the place where the two red bricks meet and attach them.
- Attach the remaining two red bricks onto the open studs of the orange brick. The red bricks should form a cross or lowercase “t” shape.
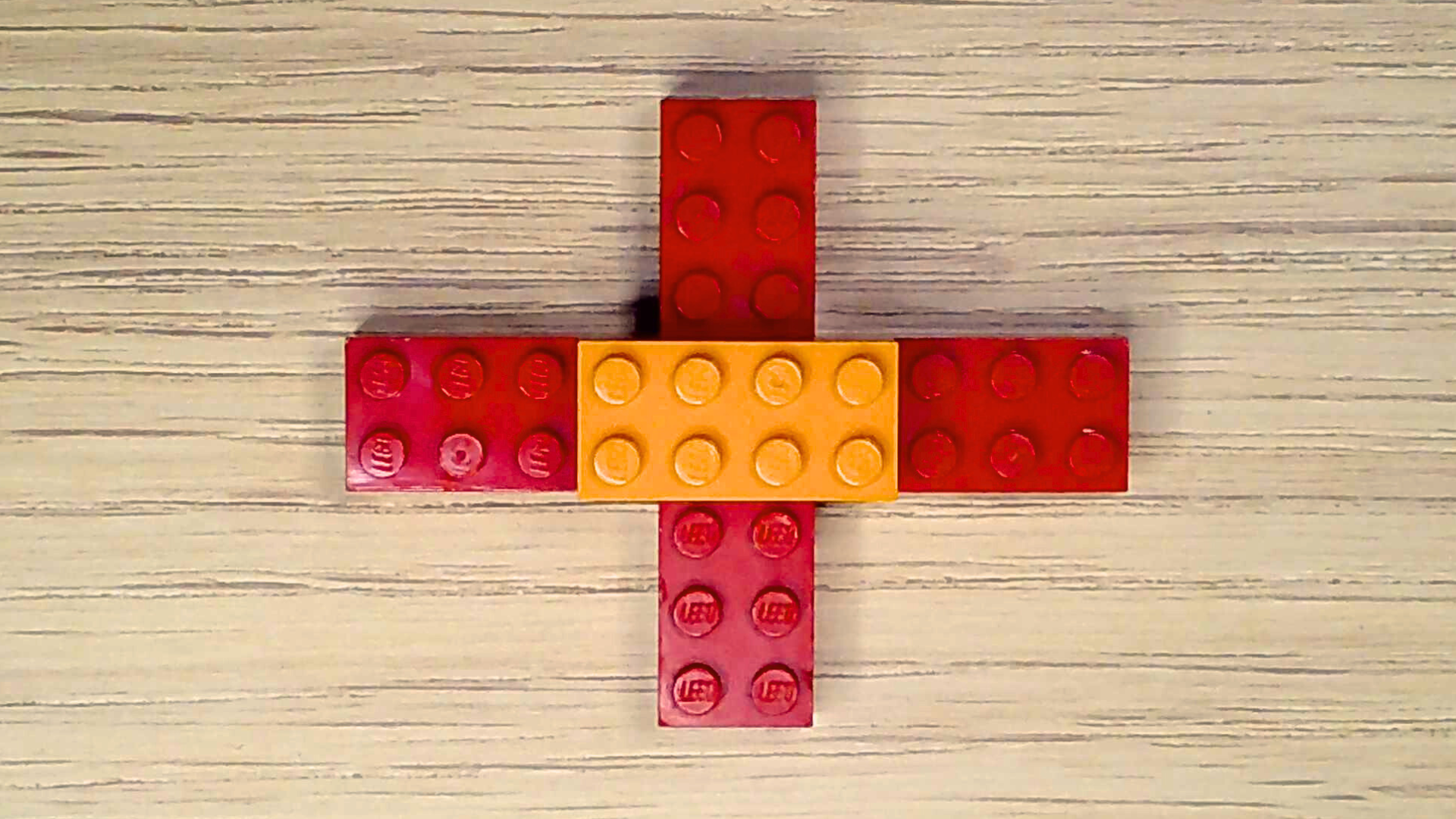
Molecule Mysteries
Now that you’ve built some model molecules, try the challenge below to test your knowledge and skills.
In the picture below, there are several different molecules: hydrogen peroxide, propane, carbon monoxide, carbonic acid, acetic acid (vinegar), and nitrous oxide. Which is which? Do the names of the molecules offer any clues?
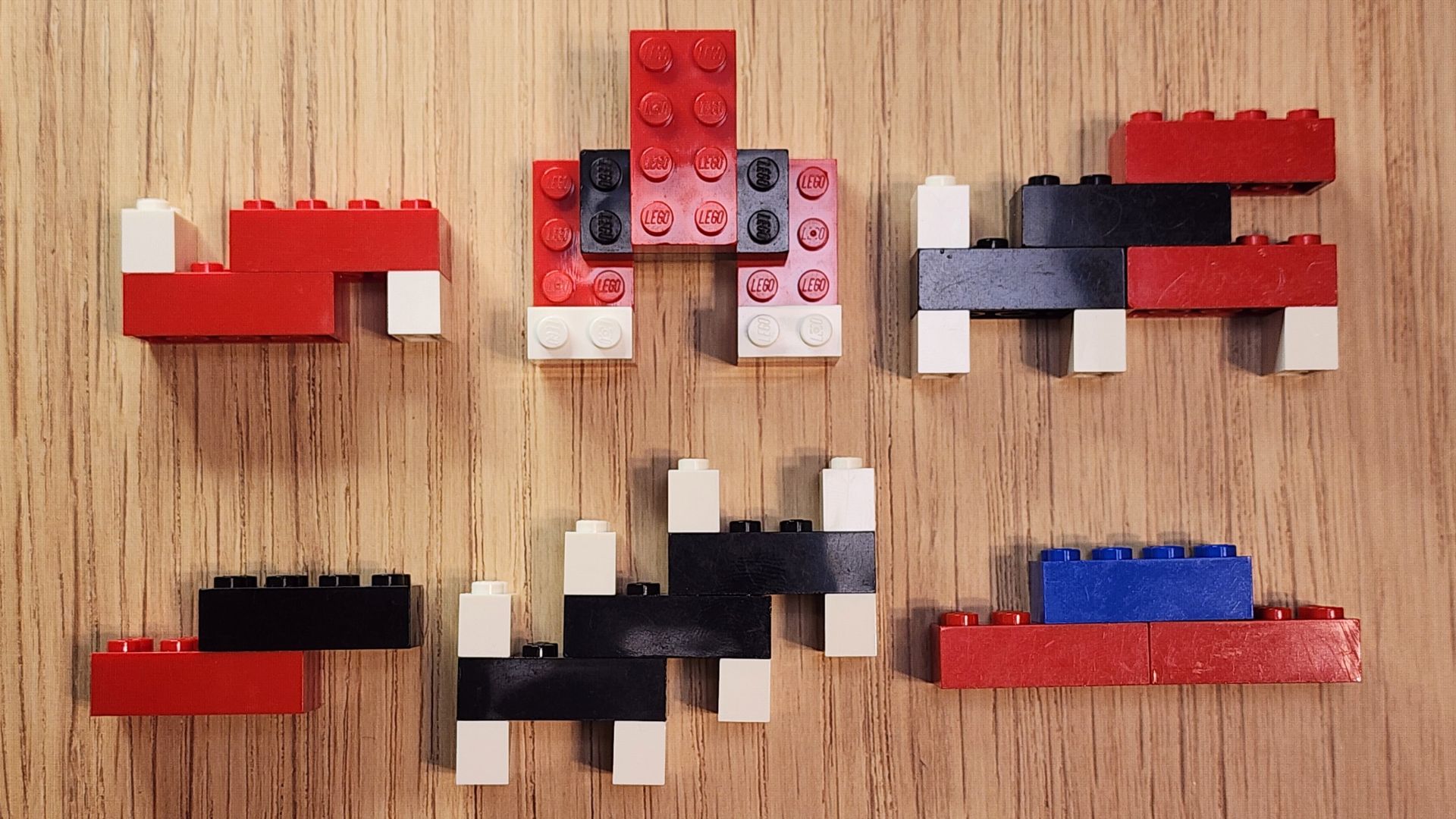
As you think about the models you have made, ask yourself:
- How are the molecules you made the same, and how are they different? What may be the reasons for those similarities and differences?
- Are there limits to how many atom bricks you can attach to a single brick? What are the limitations? Would such limitations exist in nature with chemical molecules?
- What other molecules can you make using these five elements? (You may need to do some research. See the suggestions at the end of this resource.)
How Five Elements Define Life On Earth
Elements Shaped Our Planet
In his book, Elemental: How Five Elements Changed Earth’s Past and Will Shape Our Future, author Stephen Porder argues that these five elements—your building blocks—are “life’s formula.” Ninety-nine percent of our cells are made of carbon, hydrogen, and oxygen. Meanwhile, 80% of the air we breathe is made of nitrogen. And phosphorous? It’s the key to almost every biological molecule used by every organism on Earth. Over time, the availability of these elements forced organisms to adapt to changes in the climate and environment. Even now, these elements shape life on the planet as fundamental building blocks of your cells, the soil, the air, water, and even the fossil fuels we use to make energy.
“[O]ur living planet is not just a living planet because it houses life, but because it’s shaped by life. And this interaction between the living and unliving world is really what determines the characteristics both of our planet and the organisms that live upon it.” – Stephen Porder
Keep Learning About Elements And Compounds
Want to learn more about atoms, molecules, elements, and compounds? Here are some things to try:
- Listen to the Science Friday interview with Steven Porder or read an excerpt from his book, Elemental: How Five Elements Changed Earth’s Past and Will Shape Our Future.
- Continue modeling and experimenting with MIT’s free Lego Chemistry curriculum. You can investigate oxidation, combustion, photosynthesis, and more!
- Explore all the elements with an interactive periodic table.
- See if you can memorize and sing Tom Lehrer’s “The Elements Song.” (It’s a 1967 classic, but if you want an updated version that includes the elements discovered since then, check out this version from AsapSCIENCE.)
Next-Generation Science Standards
This resource works toward the following performance expectations:
- 5-PS1-1 Matter and Its Interactions: Develop a model to describe that matter is made of particles too small to be seen.
- MS-PS1-1 Matter and Its Interactions: Develop models to describe the atomic composition of simple molecules and extended structures.
Credits
Lesson by Sandy Roberts
Copyediting by Lois Parshley
Digital Production by Sandy Roberts

The molecular modeling system described in this set of activities was created by Kathleen M. Vandiver, and additional materials and examples can be found on MIT Edgerton Center website.
Meet the Writer
About Sandy Roberts
Sandy Roberts is Science Friday’s Education Program Manager, where she creates learning resources and experiences to advance STEM equity in all learning environments. Lately, she’s been playing with origami circuits and trying to perfect a gluten-free sourdough recipe.
