Grade Level
6 - 8
subject
Physical Science
stem practices
Planning and Carrying Out Investigations
Activity Type:
physics, materials science
In this activity, students will learn about phosphorescence and how certain materials can absorb and store energy from a light source. Students will use critical thinking skills to hypothesize which type of light — incandescent, ultraviolet, infrared or fluorescent—will produce the brightest glow from a glow-in-the-dark star. Students will perform an experiment using cameras to observe the intensity of the resulting glow from each type of light source.
Phosphorescence, the phenomenon that you see when something glows in the dark, occurs when a material absorbs energy from a light source, and then continues to emit light after the light source has been removed. This glowing effect has been used for many different purposes, ranging from entertainment in the form of novelty items, to practical applications such as traffic safety signs.
Materials
- One lamp fixture
- One incandescent light bulb – 40 watts
- One compact fluorescent light bulb – 7 watts
- One ultraviolet light or black light bulb – 40 watts
- One infrared or red heat bulb – 40 watts. Available at pet stores.
- Glow-in-the-dark stars, all in the same size—four for each student or group of students
- One camera—or students may bring their own

Procedure
For this activity, the glow-in-the dark stars must lose their glow completely before you can start the experiment. Each student or group of students will need a total of four stars. In order to save time, we recommend that you separate enough stars for each student or group of students, and place them in a covered container or small bag that is impervious to light. Just before the lesson, place four stars each in individual containers or bags.
- Begin the lesson by having students watch the video above. Begin a discussion with the students on how and why some objects are able to glow in the dark. Ask students if they think that different types of light will affect how well a phosphorescent object will glow.
- Show students four different light sources: an incandescent light bulb, a fluorescent light bulb, an infrared light bulb and an ultraviolet light bulb. Warn students not to look at the infrared or ultraviolet light bulbs once they are turned on. Review with students the differences between each type of light, and ask them which type of light they think will cause a phosphorescent object to glow the brightest. Tell students they will conduct an experiment to test their theories.
- Hand out the bags/containers of glow-in-the-dark stars to each student or group of students, making sure that the stars remain enclosed. Tell students not to remove the stars from the closed containers/bags until it is time to conduct the experiment. Why is this important?
- Close the curtains/shades and turn off the lights in the room. Make sure that there is enough indirect light for students to see. Have students remove one star from the container, while you shine the infrared light on the object at a distance of two inches for 20 seconds. Remind students not to look directly at the infrared light bulb once it is turned on.
- Have students ready with a camera to take a picture of the star after the 20 seconds are up. Make sure students take a picture of the object immediately after the light is turned off at a distance of six inches and with the flash OFF. Why is it important to have the flash off?
- Repeat the experiment three more times, using another star from the container for each light source (incandescent, fluorescent, and ultraviolet). Remind students not to look directly at the ultraviolet light bulb once it is turned on. Have students write down the order of the light sources used, so that they will be able to differentiate each picture later.
- Depending on the type of camera, have students compare each picture by either viewing them on the camera, downloading pictures onto a computer, or developing the pictures. Which light source caused the star to glow the brightest?
- Have students place the images in the order of brightest to least bright. Discuss with students why they think one particular light source worked better than others.

Understanding Light Waves
When light hits normal, everyday objects, it usually is either absorbed or immediately reflected. However, when light hits a phosphorescent object, the energy is absorbed and stored or “charged.” The phosphorescent object will hold onto the energy and gradually re-emit the energy as light. (The color it glows depends on the type of material.) Because the light slowly is being released, it appears to glow even after the light source is turned off.
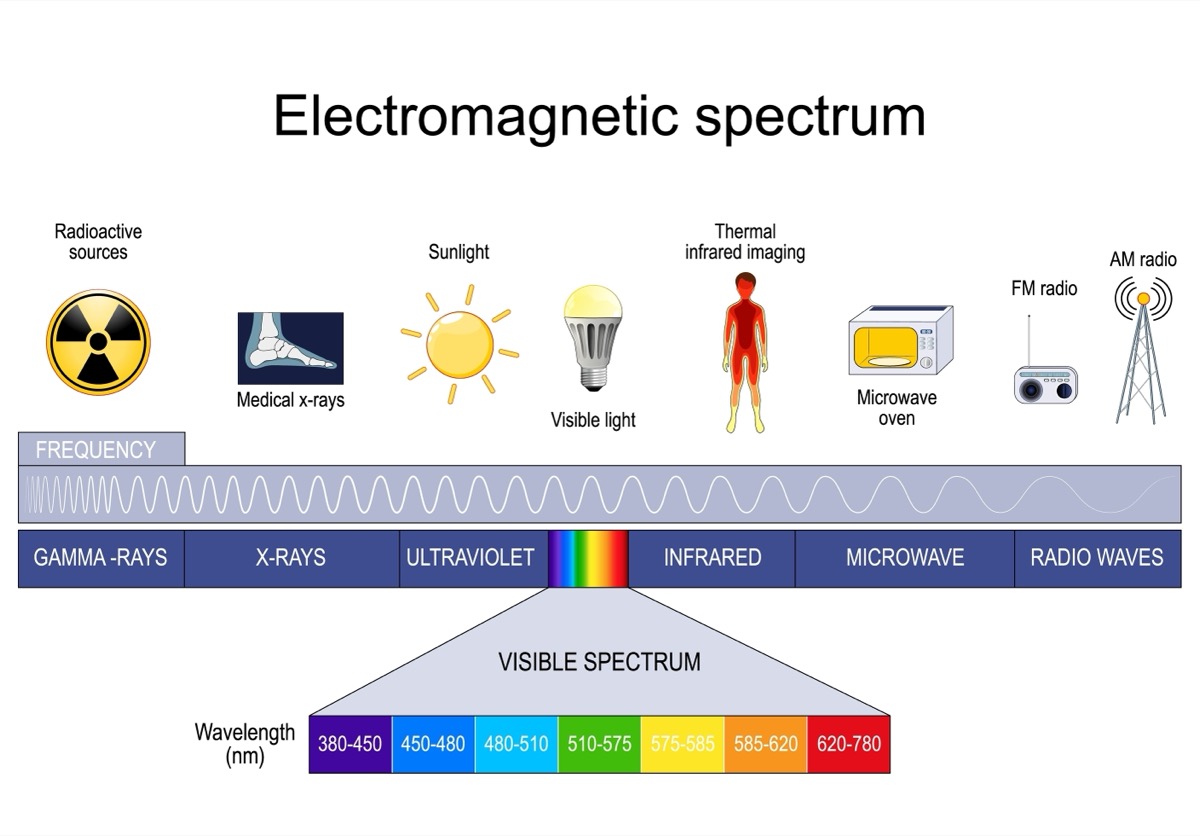
Different types of lights will affect how much energy is able to be stored in a phosphorescent object. Infrared light waves have less energy than ultraviolet light waves. Because of this, infrared light will not “charge” the object as well as ultraviolet light. Although incandescent and fluorescent lights both look white, the individual colors in them are different. Incandescent light bulbs emit visible light with a large percentage of infrared light. Fluorescent lights emit visible light with some ultraviolet light mixed in.
The stars that absorbed pure ultraviolet light will be the brightest, since ultraviolet light has the most energy out of all light sources tested. The fluorescent light contains some ultraviolet light, so it will be the second brightest.
Some questions you might ask:
- Instead of testing for intensity of light emitted, how can students test the persistence of light emitted?
- Would the results be the same if a different phosphorescent material were used?
- How does this experiment relate to the electromagnetic spectrum? Are there other types of light waves that also could be tested?
- What are some practical or important uses for phosphorescent objects?
Extended Activities and Links
- Explore fluorescence by examining classroom or household items under a black or ultraviolet light, taking care not to look directly at the light source once it is turned on. Have students conduct an investigation, based on their observations, to determine the differences between fluorescence and phosphorescence.
- Have students research the different parts of the electromagnetic spectrum and create a chart illustrating the wavelengths of each type of light wave. What are the negative or positive effects of each type of light wave on humans?
- Have a glow-in-the-dark contest in which students design and demonstrate their own unique glow-in-the-dark products.
- Build a device to measure the intensity and duration of light produced by glow sticks and other glow-in-the-dark materials:
Vocabulary
- Phosphorescence – A phenomenon in which an object absorbs light energy and gradually releases it, even after the original light source is removed.
- Visible Light – The only type of light waves that humans are capable of seeing, ranging from red to violet and including all the colors of the rainbow.
- Ultraviolet (UV) Light – A type of light that is invisible to humans and is a significant component of sunlight.
- Infrared (IR) Light – A type of light that can be experienced in the form of heat. Although we cannot see infrared without the use of a special filter, living and non-living things emit infrared light.
- Incandescence – The emission of visible light from a hot body due to its temperature. In an incandescent light bulb, a filament, or thin wire, is heated to a temperature at which a small fraction of the radiation falls in the visible spectrum. But most of the radiation is emitted in the infrared part of the spectrum, which means that incandescent lights are relatively inefficient.
- Fluorescence – The emission of light by a substance that has absorbed light of a different wavelength. In a fluorescent light bulb, electricity is used to excite a small amount of mercury vapor or gas. The excited mercury atoms produce ultraviolet light that then causes a phospher to fluorese, producing visible light. A fluorescent light bulb converts electrical power into useful light more efficiently than an incandescent bulb.
This lesson plan was created by the New York Hall of Science in collaboration with Science Friday as part of Teachers Talking Science, an online resource for teachers, homeschoolers, and parents to produce free materials based on very popular SciFri Videos to help in the classroom or around the kitchen table.
The New York Hall of Science is a science museum located in the New York City borough of Queens. NYSCI is New York City’s only hands-on science and technology center, with more than 400 hands-on exhibits explore biology, chemistry, and physics.
Meet the Writer
About Ariel Zych
@arieloquentAriel Zych is Science Friday’s director of audience. She is a former teacher and scientist who spends her free time making food, watching arthropods, and being outside.
