The Long And Short Of Telomere Activity
11:57 minutes
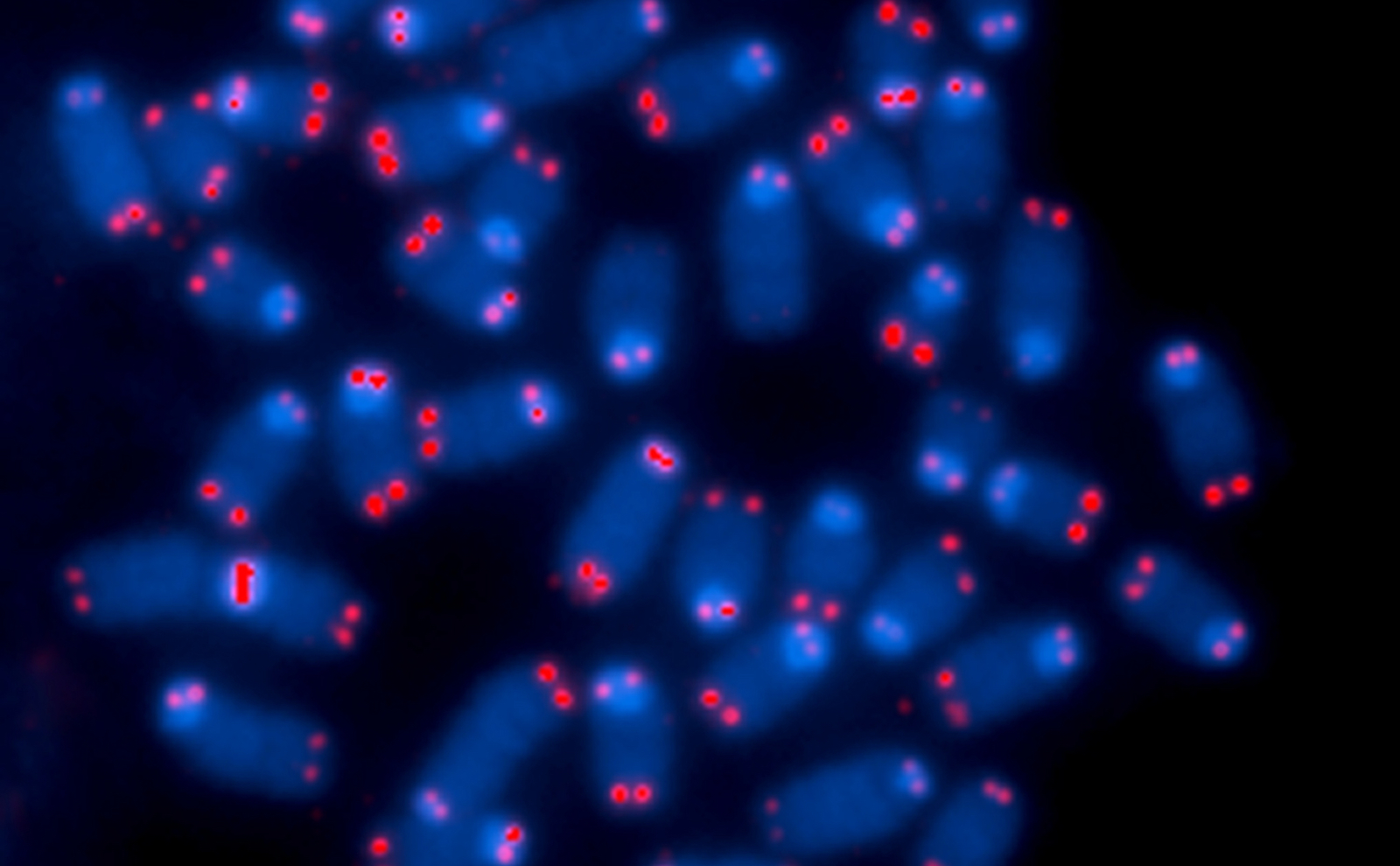
Telomeres are repeating short sequences of genetic code (in humans, TTAGGG) located on the ends of chromosomes. They act as a buffer during the cell replication process. Loops at the end of the telomere prevent chromosomes from getting inadvertently stuck together by DNA repair enzymes. Over the lifetime of the cell, the telomeres become shorter and shorter with each cell division. When they become too short, the cell dies. Telomere sequences weren’t thought to do much else—sort of like the plastic tip at the end of a shoelace.
Writing in the Proceedings of the National Academy of Sciences, researchers now argue that telomeres may actually encode for two short proteins. Normally, those proteins aren’t released into the cell. However, if the telomere is damaged—or as it gets shorter during repeated cell replication cycles—those signaling proteins may be able to leak out into the cell and affect other processes, perhaps altering nucleic acid metabolism and protein synthesis, or triggering cellular inflammation.
Jack Griffith, one of the authors of the report and the Kenan Distinguished Professor of microbiology and immunology at the UNC School of Medicine, joins SciFri’s Charles Bergquist to talk about the idea and what other secrets may lie inside the telomere.
Dr. Jack Griffith is the Kenan Distinguished Professor of Microbiology and Immunology at the UNC School of Medicine in Chapel Hill, North Carolina.
FLORA LICHTMAN: This is Science Friday. I’m Flora Lichtman.
CHARLES BERGQUIST: And I’m Charles Bergquist. At the end of each of your chromosomes is a repeating chunk of six DNA letters called a telomere. It’s often described as being sort of like the plastic tip on the end of a shoelace, a thing that protects the more important part of the strand but doesn’t do much else.
And as your shoelace or chromosome gets older, those ending tips get worn down and damaged. But recently, scientists reported that instead of being just silent placeholders on the chromosome, telomeres may actually encode for small proteins. And proteins do things. They change things, perhaps helping your cell run normally or perhaps promoting diseases, such as cancer.
Dr. Jack Griffith is the Kenan Distinguished Professor of Microbiology and Immunology at the UNC School of Medicine in Chapel Hill, North Carolina. He’s one of the authors of a report on the finding in The Proceedings of the National Academy of Sciences. Welcome to Science Friday, Dr. Griffith.
JACK GRIFFITH: Thank you so much, Charles.
CHARLES BERGQUIST: So if telomeres can encode for these two small proteins, do we know what these proteins do in a normal cell?
JACK GRIFFITH: We don’t. And this is all so new and, I might say, paradigm shifting or shocking, that the field is really, perhaps, reeling from this discovery. And we’re going ahead as much as we can to find out what these two proteins do because telomeres were discovered 80 years ago as some mysterious elements at the end of the chromosome, which keeps two different chromosomes from sticking end to end. But nobody knew how that was done until six decades later when my colleague Tish Long at the Rockefeller and I show the end actually isn’t an end. It’s a little circle, a loop.
So it’s an endless end. And that keeps the chromosomes from sticking end to end. And we call these T-loops. But it was assumed that this DNA, as you mentioned, is rather boring. TTA-CCG over and over again. And it wouldn’t encode RNA or proteins. And that’s been the dogma.
CHARLES BERGQUIST: As you say, this is surprising because these sequences weren’t thought to do very much beyond act as this buffer. So how did you find this?
JACK GRIFFITH: Well in 2007, a group in Switzerland showed that telomeres actually do something. And they’re transcribed into RNA. And it turns out this RNA is like Velcro. It sticks to chromosomes. And it kind of globs up to the ends of the telomeres. And it never, never, never gets out into the cytoplasm.
But again, because it’s a simple repeat, it doesn’t look like a protein coding sequence. And no one thought it would do that. But in a Star War analogy in a galaxy far, far away with the world of repeated nucleotide diseases, like ALS, frontotemporal dementia, myotonic dystrophy, Fragile X, and there’s two fabulous scientists in that galaxy, Norah Random and Maurice Swanson.
And they discovered that the ALS disease involves an RNA that has six repeats, CCG-TTG. And that, by an unusual mechanism, can be turned into proteins. And since I know those people very, very well because I lived in that galaxy years ago, I wrote down CCG-TTG and then underneath it, TTA-TTG, and said, wow, If the ALS RNA makes these two very toxic kind of proteins that they discovered, what would telomeres do?
It looks like it makes the same thing. So telomeres make two proteins, one’s very hydrophobic and makes little prion-like rods and amyloids. The other is very charged, binds nucleic acids. And in the ALS world, they’re different amino acids, but they’re basically the same, very hydrophobic. And then one’s very charged.
And so that was a shock 10 years ago. And we made the proteins chemically, looked at them in my electron microscope, did as much as we could. But it was not until 2020 that I was able to recruit a fabulous postdoctoral fellow, Dr. Taghreed Al-Turki, who had done her graduate work at Colorado State and had expertise in light microscopy.
And so we made an antibody to one of the two proteins. Taghreed took over. And due to her fabulous work, we have been able to show that this one protein in particular is present in all human cells that we can detect. It’s much higher in a number of cancer cells. It’s higher in cells from people with certain telomere diseases.
And recently, we’re finding it higher in serum from people with some cancers. So that’s really quite exciting but very new.
CHARLES BERGQUIST: Yeah, so as cells age and divide, the telomeres get shorter. Does that shortening process alter the protein that you found at all or make you produce any less of it or more of it?
JACK GRIFFITH: I think it actually makes more of it because as the telomere shortens, it’s kind of a self-emulation of the telomere as you get older and older, eventually the telomere get so short it cannot make our little T-loops. And now the telomere is called dysfunctional.
And now this RNA that is very carefully kept away in a nucleus can get out into the cytoplasm and make these two proteins, signaling proteins. And so those proteins can then shut down cellular processes. We think that they may turn down protein synthesis. They may get out into little things called exosomes that are shed from cells. And they could communicate the kind of distress that cell A is undergoing to cells B, C, and D all around them.
CHARLES BERGQUIST: Researchers have talked about telomeres as being a kind of marker of cellular aging. Does finding these new proteins teach us anything about aging in general?
JACK GRIFFITH: That’s actually a question that we’re starting to think about in detail because we have reason to believe that these proteins are actually fed into our blood in the serum. And so we’re working on– our big effort in the laboratory right now is to work on a blood test for these proteins in serum.
And our guess is that as you age, that level would slowly, slowly, slowly get higher and higher and higher. And the older you get, it would go up and up and up. The cells go into senescence, become inflamed, and so forth.
But in certain cases, like, say, younger people who do not know that they have a disease called idiopathic pulmonary fibrosis, which is a disease or telomeres, and they have very short telomeres, but it makes more of this telomeric RNA. Those people might have a big spike of this VR protein that were assaying early in life.
And so if you discovered that, you could then identify those people. And they would know they’re at risk. And so there are certain things they could do during life to ameliorate the symptoms of that disease.
CHARLES BERGQUIST: So even normalizing for age, all human telomeres aren’t the same. My telomeres may have started off at a different length from yours.
JACK GRIFFITH: That’s absolutely true. So people have different telomere lengths. And they change during their lifetime. And it may be, in part, inherited.
I just had lunch with one of our veterinary pathologists who was talking about having cloned pigs. And they cloned pigs from another pig that was somewhat older. And now they’ve discovered that their new cloned pigs are dying very early because their telomeres are getting short. And they just started out kind of short-changed. The baby pig, the piglets were short-changed because they started out with short telomeres. And so there are probably people who start out that way.
There are also rare diseases you find in children that are also involved in defects in the telomeric machinery. And these people often pass away at an early age because their telomeres just are not functioning or are unusually short.
CHARLES BERGQUIST: We keep talking about problems associated with extra-short telomeres. Is there any harm or any disadvantage in people that have super-long ones?
JACK GRIFFITH: Yes, there’s been a big, big study of that. One of the best studies was in Denmark, where they have the ability to look at a whole nation’s worth of people. And they found that on average, people that have shorter telomeres tend to have trouble with cardiovascular disease at an earlier age because the heart muscle cells are now dying at an early age, whereas people who have very long telomeres have less susceptibility to cardiovascular issues but they have higher risk for cancer because our most fundamental barrier against cancer are the telomeres getting shorter and shorter and shorter and then the cells quit replicating.
And it’s only those few cells that jump over that barrier become cancerous. So if you have a really long telomere, there’s more chance that those cells will divide and then become cancerous than if you start out with shorter telomeres.
CHARLES BERGQUIST: So in some cases, it’s advantageous to having almost a kill switch for an aging cell?
JACK GRIFFITH: Absolutely, yes.
CHARLES BERGQUIST: Interesting. So as you and your colleagues work to try and figure out what these two proteins may do, is this just sort of another strike against the term junk DNA?
JACK GRIFFITH: Oh, yes, yes. Taghreed and I keep laughing in the lab, because this RNA that makes these proteins in the literature is called a long noncoding RNA. And it’s in all the papers. And we keep saying that we have forced the field to get rid of that term for this RNA.
And it’s going to take time. I have colleagues that are still, maybe, maybe not. They want to see more data. And we really appreciate that. But we have a series of three papers right now kind of outlined that I think are going to put a lot more meat on the story.
And that’s what science is all about. You come up with a new idea. People, well, maybe, maybe not. But you just keep piling on more and more findings, then hopefully it will turn into an exciting story. There are no stories that are important that are just start and finish in one paper.
CHARLES BERGQUIST: Well, I wish you good luck with your future research. Dr. Jack Griffith is the Kenan Distinguished Professor of Microbiology and Immunology at the UNC School of Medicine in Chapel Hill, North Carolina. Thanks so much for being with me today.
JACK GRIFFITH: Well, Charles, it’s all my pleasure.
Copyright © 2023 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/
As Science Friday’s director and senior producer, Charles Bergquist channels the chaos of a live production studio into something sounding like a radio program. Favorite topics include planetary sciences, chemistry, materials, and shiny things with blinking lights.