A Possible Achilles Heel For Troublesome PFAS Chemicals
7:43 minutes
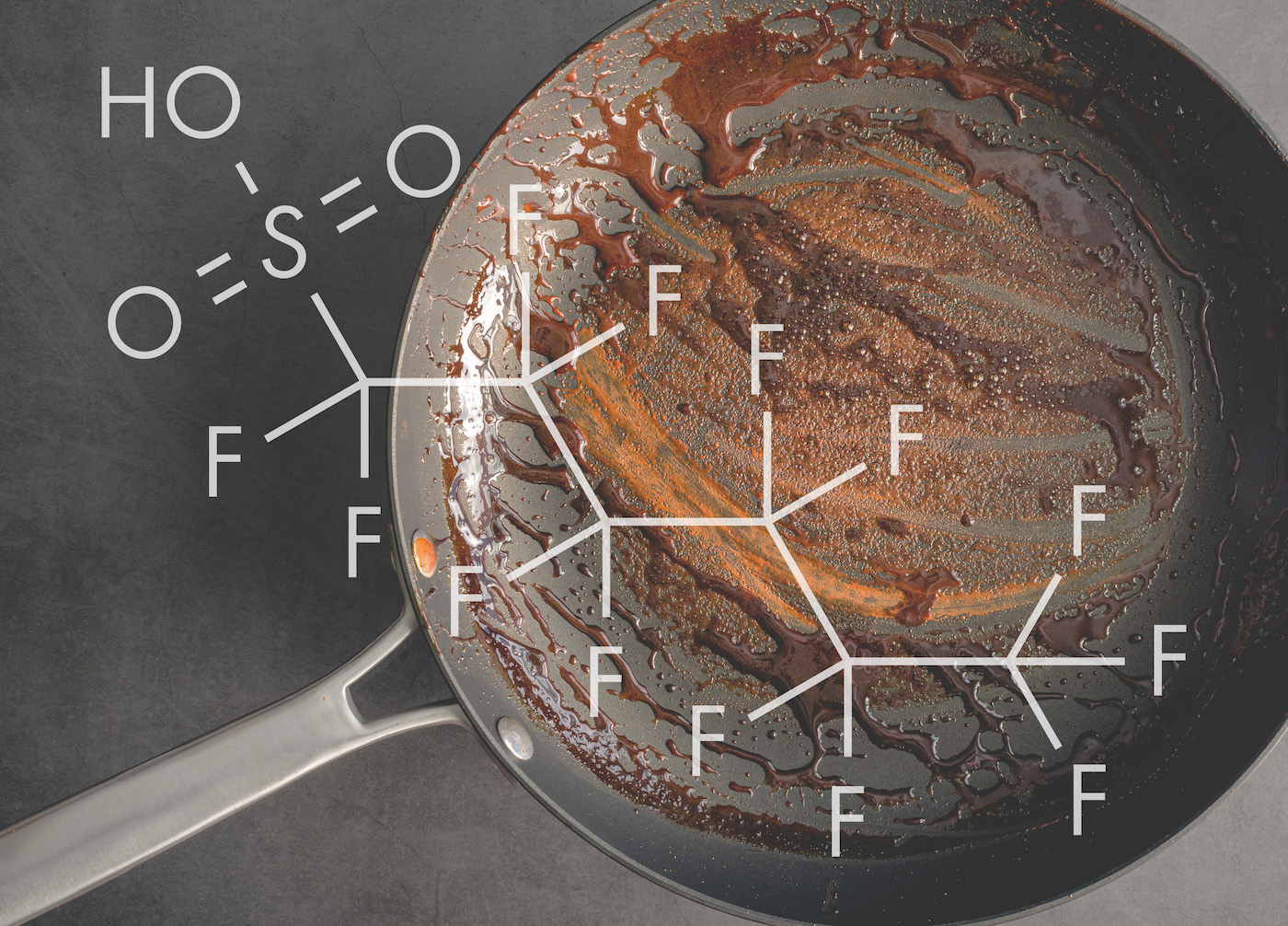
Long-lasting chemicals known as per- and polyfluoroalkyl substances, or PFAS, are used widely in everything from firefighting foam to microwavable popcorn bags. The chemicals are a popular component of polymer coatings that resist heat, grease, stains and water.
PFAS compounds (a family that includes roughly 12,000 different substances are often called “forever chemicals” in popular science coverage, because they’re designed to be super stable and don’t break down in the environment. But what makes them last “forever” from a chemistry perspective? And what can we do about it?
Current PFAS disposal methods are expensive and labor-intensive, blasting the chemicals with temperatures over 1,000 degrees C in a high-pressure environment. But a research study published in Science has found a possible Achilles’ heel: a weak spot in the chemical bonds. The research points to a new possible method for disposing of PFAS chemicals, which uses special reagents to knock off a group of oxygen atoms at the tail end of PFAS, triggering a cascade of reactions that breaks the PFAS chemicals down into harmless components.
The paper’s lead author, Brittany Trang, joins guest host Shahla Farzan to discuss this development in PFAS research.
Invest in quality science journalism by making a donation to Science Friday.
Brittany Trang is a science reporting fellow at STAT News in Boston, Massachusetts.
JOHN DANKOSKY: This is Science Friday. I’m John Dankosky.
SHAHLA FARZAN: And I’m Shahla Farzan. You may be familiar with the acronym PFAS, an umbrella term for a whole bunch of chemicals that are meant to last a long time– so long, in fact, that their nickname is “forever chemicals.” PFAS compounds are used in a ton of things, from firefighting foam to microwaveable popcorn bags. Because these chemicals are made to last a long time, they’re also really hard to break down. And that’s a problem as we learn more about how bad they are for our bodies.
But new research points to a possible Achilles heel in some PFAS chemical bonds. And that could make it much easier to dispose of these chemicals and turn them into something harmless. Joining me to talk about this is the lead author on that paper, Dr. Brittany Trang, a chemist and also a science reporting fellow at Stat News, based in Boston, Massachusetts. She did this research as part of her PhD at Northwestern University. Welcome to Science Friday.
BRITTANY TRANG: Hi. Thanks for having me.
SHAHLA FARZAN: Thanks so much for joining us. So when we talk about PFAS compounds, they’re in a lot of different things, like nonstick pans and other things that you might have around your house. Why are we using them in so many different products?
BRITTANY TRANG: It’s because they have a lot of really useful properties. So the reason that we can’t find replacements for them is because they have unique chemical properties that nothing else has, which derives from their carbon-fluorine bonds, which is what gives them their name and also gives them all these really awesome properties like being hydrophobic, so water repelling, being oleophobic– oil repelling– being really thermally stable, which is why they’re in firefighting foams. All of these things give them properties that we use in all of the really cool products that modern science has given us. But unfortunately, these have come at a little bit of a cost.
SHAHLA FARZAN: So what is it about PFAS compounds chemically that makes them so hard to break down?
BRITTANY TRANG: So all of those really cool properties that I was talking about derive from these carbon-fluorine bonds that give per- and polyfluoroalkylated substances. So because– you might learn in high school chemistry that fluorine is the most electronegative element– it wants electrons really bad– and carbon is a pretty polarizable element, so it is relatively willing to share its electrons. So when fluorine and carbon are together, the fluorine will grab onto carbon’s electrons and does not want to let go. So this creates a really strong bond that gives these compounds thermal stability. But it also gives it all of these properties of not wanting to bond with other things and the bond not wanting to break, which makes them hard to break down.
SHAHLA FARZAN: OK, so that difference in electronegativity is kind of the recipe for a really, really strong bond, then.
BRITTANY TRANG: Yeah.
SHAHLA FARZAN: So you’re the lead author of this paper that found a possible Achilles heel in some PFAS compounds. Walk us through what you found.
BRITTANY TRANG: Yeah, so past research into how to degrade these forever chemicals has focused on the fact that we know this carbon-fluorine bond is really strong. And if we want to break it, we should just put a lot of energy into the system, which does work in some cases, somewhat, but often leaves partially degraded PFAS or harder to break down PFAS because it’s just very non-targeted. How we entered this space was saying, hey, we’re chemists, and we know a lot of ways to react certain kinds of functional groups, certain kinds of groups on these chemicals. So how do we apply that to this problem?
And most PFAS have a head portion and a tail portion. So the tail portion is where all these carbon-fluorine bonds are. And the head portion is different for different PFAS, but in the case of perfluorocarboxylic acids, which we were looking at, there’s a carboxylic acid, which is a carbon bonded to two oxygens.
And so we figured out a way to pop off the head of the molecule, so that carboxylic acid portion. And then we found further ways to degrade the tail of the molecule, where, after you’ve beheaded this molecule, then the tail is actually more reactive. And we found that using sodium hydroxide broke these tail fluoroalkyl species down in a lot more mild conditions than previously thought possible. Instead of at, like, thousands of degrees, we can do it at 40 degrees Celsius, which I am told is the temperature of a hot yoga room.
SHAHLA FARZAN: OK, yes, hot yoga studios– notoriously uncomfortable. And now, when I think about them, I’m also going to be thinking about breaking down PFAS chemicals. OK, so you’re applying these two different chemicals, relatively common reagents. What does the PFAS compound break down into later?
BRITTANY TRANG: Yeah, so this is a really important part of PFAS degradation because you can see the initial compound go away, and some studies are like, yay, we made that go away. But they have no idea what it turned into. And what you want to see is all the fluorine go to fluoride, kind of like the same stuff that you have in your toothpaste.
And so we see over 90% of our fluorines go to fluoride, and the rest of it will eventually pretty much break down into that. And then, all the carbon in the molecule breaks down into various kinds of mostly carboxylic acids that are benign to humans. So these different products you can find in vegetables, you can find sold as skin care products. So these are all not harmful to humans.
SHAHLA FARZAN: So this sounds promising, but do you think that this process could potentially be used on a bigger scale, like a mass scale, to start disposing of PFAS chemicals?
BRITTANY TRANG: Unfortunately, this is where we have to tell people to hold their horses a little bit. Our method right now is not optimized at all. And somebody is going to have to figure out how to optimize it if we want to use it on a larger scale. And in that process of optimization, we probably will have to learn more about the roles of the different reagents in our degradation process. We don’t know enough to figure out, oh, how can we use less of this solvent, or how do we maximize the efficiency?
But I think what the most exciting thing is would be if other chemists might take a look at the principles that we discovered were active in this destruction, which people previously thought was not possible, and if those principles get used in other people’s work that is probably a lot more industrially friendly than ours is.
SHAHLA FARZAN: So much on the horizon for this field of research. Well, we’ll have to leave it there. I’d like to thank my guest, Dr. Brittany Trang, chemist and also a science reporting fellow at Stat News, based in Boston, Massachusetts. She did this research as part of her PhD at Northwestern University. Thanks so much for joining us, Brittany.
BRITTANY TRANG: Thank you for having me.
Copyright © 2022 Science Friday Initiative. All rights reserved. Science Friday transcripts are produced on a tight deadline by 3Play Media. Fidelity to the original aired/published audio or video file might vary, and text might be updated or amended in the future. For the authoritative record of Science Friday’s programming, please visit the original aired/published recording. For terms of use and more information, visit our policies pages at http://www.sciencefriday.com/about/policies/.
Kathleen Davis is a producer and fill-in host at Science Friday, which means she spends her weeks researching, writing, editing, and sometimes talking into a microphone. She’s always eager to talk about freshwater lakes and Coney Island diners.
Shahla Farzan is a science journalist, PhD ecologist, and editor with American Public Media, where she helps produce science podcasts for kids. She loves showcasing the many weird and wonderful aspects of science—and encouraging young, curious thinkers to question and explore the world around them.