Grade Level
6 - 8
minutes
1- 2 hrs
subject
Chemistry
stem practices
Planning and Carrying Out Investigations
Activity Type:
holiday activity, Household materials, Kitchen experiment
Background
There are so many lovely harbingers of spring: buds on trees, robins pecking for worms, and Easter egg hunts. Everyone loves the thrill of spotting a brilliantly colored Easter egg hidden in the grass. But, before hiding, that egg needed a dye job. That’s where we turn to chemistry.
How Dyes Work
It starts with how we color foods. Synthetic dyes, like food coloring, use organic molecules. When white light hits a molecule’s electrons, some wavelengths of light (colors) are absorbed, while others are reflected. Whatever color of light gets reflected is what we see. Subtle differences in molecular structure will drastically change a dye’s color. Check out the diagram below to see how this light absorption and reflection works.
S. O’Malley
When we apply a chemical dye to the surface of an object, we modify and rearrange the molecules on that surface. Doing this changes the wavelengths of light that the object typically absorbs and ultimately changes the color that the object appears to be. For example, a simple substitution of a hydrogen atom (H) with a hydroxyl group (OH) will change a blue-reflecting molecule into a green one.
Why Add Vinegar to Egg Dye?
Most instructions for dyeing an egg say to add vinegar to the dye mixture, but why? When an egg is soaked in an acidic mixture, two things happen. First, the eggshell reacts with the acid and produces carbon dioxide gas. (That’s why bubbles form on the surface of the eggshell while it soaks.) The shell then starts to dissolve, which increases the surface area of the egg and exposes more of the egg to the dye.
Second, proteins in the thin layer of the eggshell’s cuticle react with the acid. The proteins become protonated (i.e., they acquire extra hydrogen ions), which means that more positive charges collect on the shell’s surface. Those positive charges easily bind to the dye molecules, which are negatively charged (opposites attract!), and the dye sticks to the egg surface.
Will Other Acids Work?
In general, acids are chemical compounds characterized by their ability to produce a surplus of positively charged hydrogen ions. Ordinary white vinegar is a dilute solution of acetic acid, and is only one example of a common household acid. You also find acids in fruit juice, soda, and some pain relievers. A dye mixture can be made more acidic by using a greater amount of acid or by using a stronger acid.
Quantitatively, acids are stronger if they have a larger acidity constant, or Kavalue. This means they give up their hydrogen(s) more readily in solution. In the following experiment, you will use various household acids like the ones below to determine how acid strength affects the color of dyed eggs.
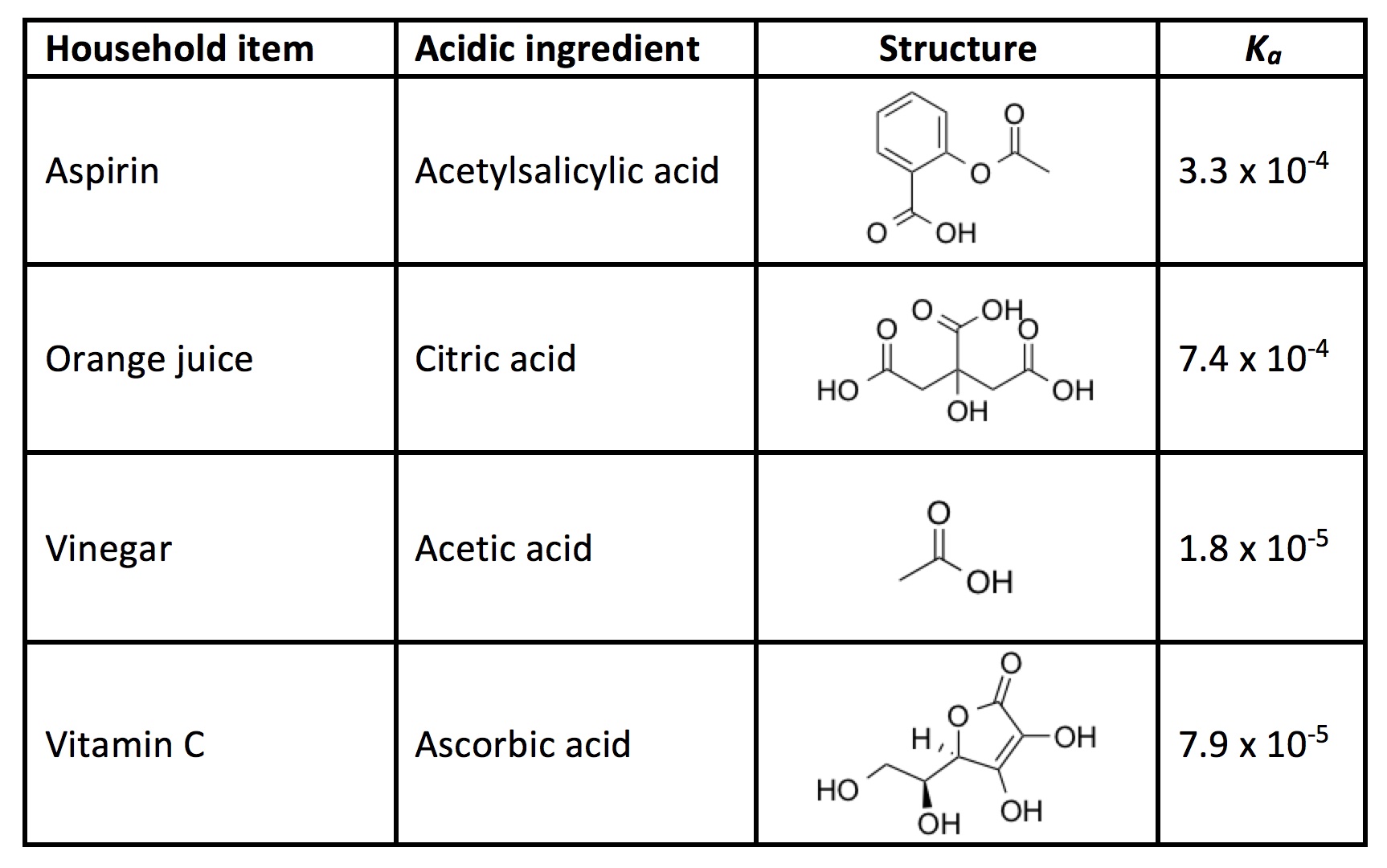
Activity
What happens if eggs are dyed using coloring agents and various household acids like fruit juice, soda water, aspirin, and vitamin C tablets? Does the acidity of a dye mixture affect the intensity of the color? Can we impart any color we want, simply by mixing the right combination of dye and acid?
Materials
(8 x 5 = 40 possible combinations)
- Four dozen white eggs, brought to room temperature
- 40 plastic cups (one for each possible egg x acid combination)
- Food coloring (eight different colors)
- Four household acids (e.g. vinegar, aspirin, vitamin C, fruit juice)
- Hot tap water
(3 x 4 = 12 possible combinations)
- One dozen white eggs, brought to room temperature
- One dozen plastic cups (one for each possible egg x acid combination)
- Food coloring (three different colors)
- Three household acids (e.g. vinegar, aspirin, vitamin C, fruit juice)
- Hot tap water
Procedure
- Arrange the plastic cups in a grid to test different colors and acids. The size of the grid will depend on the number of acid–color combinations you plan to test. If you want to test three colors with four different solutions (three acids and one control of just water), you’ll need a 4×3 grid of 12 cups. The images below show an experiment testing eight different colors, with four acids and one “control” of water, so the experimenter used an 8×5 grid of 40 cups!
- Add a small amount of a household acid to each cup (e.g., 1 Tbsp vinegar, 1 Tbsp orange juice, 1 aspirin tablet, 1 vitamin C tablet) for each color. Keep one row free of any acid to act as a control group.
- Fill a large pitcher with hot tap water. Then distribute the water to each cup.
- Add 8-10 drops of a different food coloring to each cup. Stir the dye mixtures with a spoon, rinsing the spoon each time. DO NOT allow the dye or acid from one cup to get into another. Next, add one egg to each cup.
- Soak the eggs in dye for two hours.
- Remove the eggs with a spoon. Rinse them with tap water, and lay them out to dry. To keep track of which egg came from which solution, you can arrange the eggs in the same grid formation you used to keep track of dyes and acids while they were in cups.
Results
Because different household items have a range of acidity, you probably noticed that some acidic solutions produced a more intense color than others. Which acids gave the dyed eggs the boldest and most concentrated color? Are the results what you expected?

Follow Up
You already know that the more acidic dye mixtures make more intense colors. In chemistry, reactions are affected by the concentration of the reactants involved. Now, play around with the experiment. What changes could be made to the dye mixture that would alter the outcome? How would adding common household bases, such as baking soda or ammonia, change the color? What would result from using different temperatures or times for the reaction?
Try Some Natural Dyes!
Eggs can be dyed with natural dyes from color-rich foods instead of synthetic dyes from food coloring. Boiling pieces of red cabbage, beets, or turmeric powder in hot water and decanting the liquid gives a homemade version of food coloring that can also be used for egg dyeing.
Photographs are courtesy of Steven O’Malley and Jeff Potter.
Meet the Writer
About Steven O’Malley
Steven O’Malley is a chemistry teacher at Stuyvesant High School in New York City and a Master Teacher Fellow at Math for America. He earned his PhD in organic chemistry from Columbia University. He enjoys running, learning new languages, and helping students learn about science.
3 thoughts on “Eggs To Dye For”
Comments are closed.


Appears from the comparison photo that vinegar results in by far the deepest, most saturated coloring.
that was pretty cool
Fun experiment!
Are all of the eggs safe to consume after being dyed? (Specifically asking about the aspirin and the length of time they sit in the solution.)