The Plastic Battery That Doesn’t Explode
The inventor thinks the new technology could revolutionize the future of batteries.
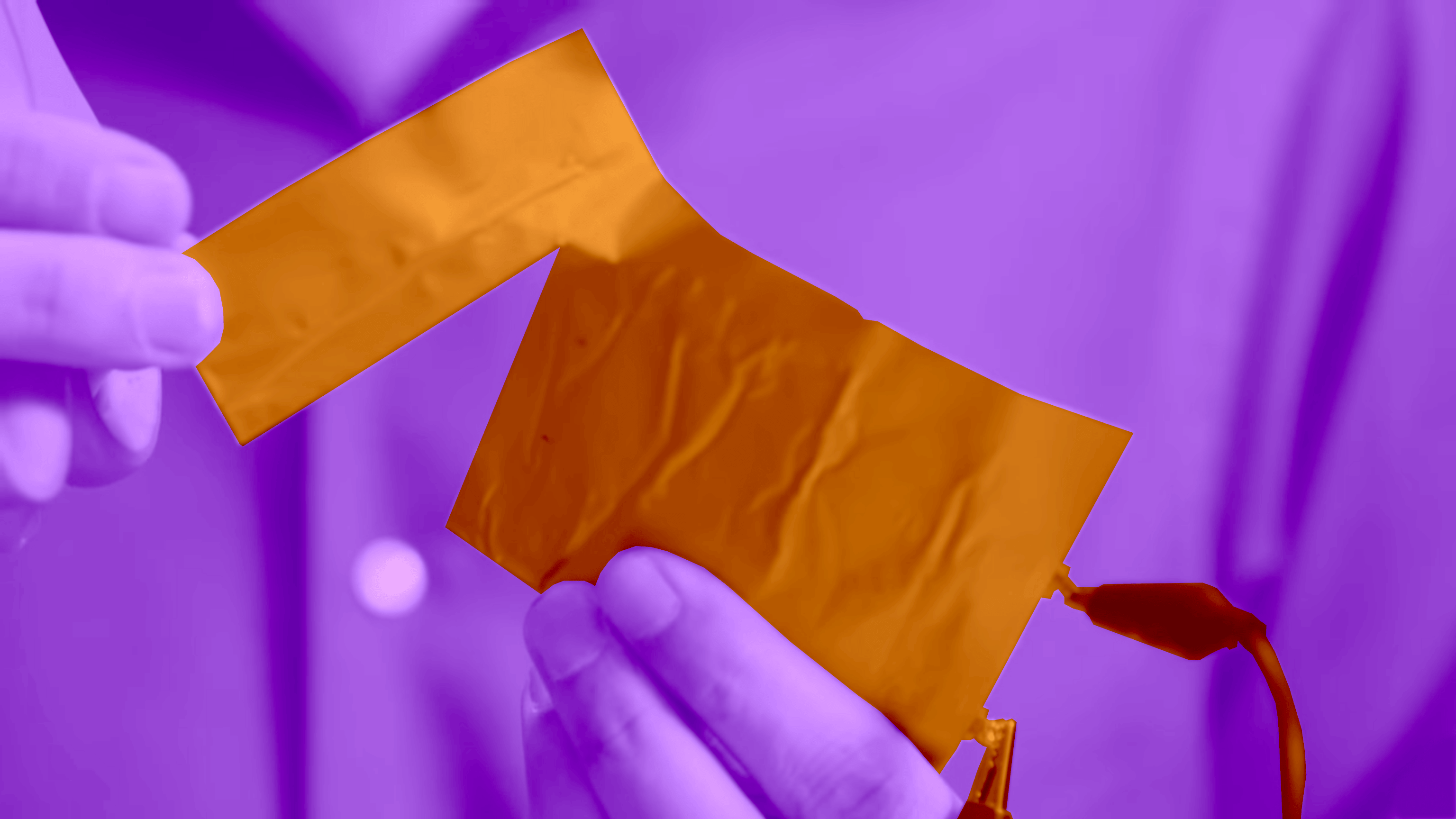
Lithium-ion batteries have been the culprits behind a string of combusting devices. They’ve caused hoverboard fires, Samsung Galaxy Note 7 smartphone explosions, and, most recently, a major recall of HP laptops. What makes these batteries so volatile is a highly flammable liquid called a liquid electrolyte.
“You can think of it as kerosene—like you’re walking around with kerosene in your smartphone, in your purse, and in your pocket,” says Mike Zimmerman, a materials science professor at Tufts University, and founder and CEO of the battery company, Ionic Materials.
Zimmerman has developed a safer electrolyte—specifically, a solid-state, plastic electrolyte—that won’t pose a fiery risk. He and a team of engineers and scientists at Ionic Materials, based in Woburn, Massachusetts, have put the concept to the test by building the first successful plastic electrolyte battery that can work at room temperature.
The ubiquitous lithium-ion battery has been around since 1991. Over the decades, scientists have been grappling with three vexing problems: These batteries are explosive, expensive, and have limited energy capacity. In a recent segment on Science Friday, David Pogue, a technology journalist and host of the NOVA documentary “Search for the Super Battery,” discussed different options that could replace the lithium-ion battery, from saltwater batteries to ice batteries. He concluded that Ionic Materials’ plastic battery was one of the most promising.
A battery is made of three primary components: a positive and negative electrode, known as the cathode and anode, which are separated by a chemical barrier called the electrolyte. The electrolyte is like a highway for ions, explains Zimmerman—it allows them to flow between the anode and cathode.
There are various ways that lithium-ion batteries can combust. For instance, overcharging, as well as contact between the anode and cathode—perhaps due to a manufacturing defect—can cause an electrical short.
“When you get a short, things heat up,” says Zimmerman. “When [the liquid electrolyte] reaches a certain temperature, it just starts burning.”
According to Zimmerman, researchers have been experimenting with two types of solids to replace the liquid electrolyte: ceramics and plastics. He found that ceramics were brittle and difficult to make on a large scale, while previous plastic prototypes could conduct ions, but only at very high temperatures.
Zimmerman’s team at Ionic Materials developed a plastic polymer electrolyte that could permit the flow of ions at room temperature. It functions in the same manner as the liquid electrolyte, Zimmerman explains, but the plastic is flame-retardant, so there’s no possibility that the battery will explode.
Pogue demonstrated its safety by chopping up one of the plastic batteries—which, in this case, was very thin—with a pair of scissors while it was powering a panel of LED lights. Much to Pogue’s relief, there was no burst of flames. As he continued to cut away, the LED lights surprisingly remained illuminated.
“That was an unintended consequence,” says Zimmerman. “We were trying to make it safe. We weren’t really focusing on making it work after it was damaged.”
The battery that Pogue whittled down still worked because it has a high energy density, thanks to the incorporation of a lithium metal anode. Batteries made with lithium metal can store twice as much energy per volume than lithium-ion batteries, according to Zimmerman, but they’d be much more dangerous if used with a liquid electrolyte. Plastic eliminates the problem.
“I use my smartphone a lot, and by four o’clock in the afternoon, I need to recharge my battery,” says Zimmerman. “If we’re able to use our plastic and put in higher-energy anodes, the phone could last two or three times longer before you have to charge it up again.”
Zimmerman hopes that we’ll see devices supported by Ionic Materials’ plastic battery in two or three years. Currently, the company is working on cementing business partners and navigating the challenges of high-volume manufacturing in an industry that has become largely accustomed to producing lithium-ion batteries. His plan is to introduce the battery into smartphones and consumer electronics first, and eventually expand to electric vehicles.
“We want to have a safe battery that can put out more capacity—more energy—so people can get a lot more range per charge” in their electric vehicles, he says.
Lauren J. Young was Science Friday’s digital producer. When she’s not shelving books as a library assistant, she’s adding to her impressive Pez dispenser collection.